2025 AIChE Annual Meeting
(253h) Electrochemical Reduction of CO2 on Metal Oxide/Copper Interfaces: Comparisons of Findings from Constant Electrode Potential Vs. Computational Hydrogen Electrode Models
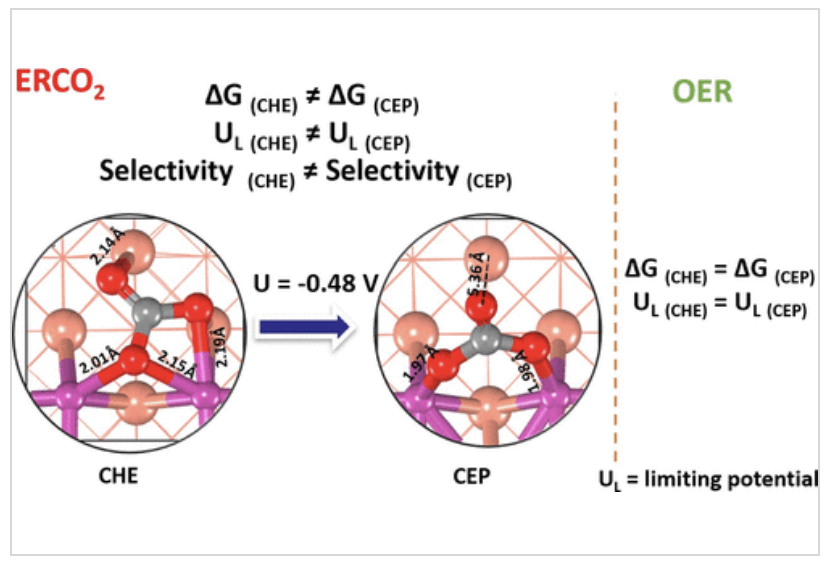
Computing limiting potential and product selectivity with reasonable accuracy is crucial for computational electrochemical studies. In these studies, the applied potential (U) is incorporated either by adding |e|U to the reaction free energy (computational hydrogen electrode, CHE model) or by using a self-consistent procedure (constant electrode potential, CEP model). We investigated ERCO2 to CH4(g)/CH3OH(l) and OER on metal-oxide/copper (MO)4/Cu(100), M = Fe, Co and Ni) catalysts using both CHE and CEP models, comparing potential limiting steps and product selectivity. Our results show that the CEP model predicts different product selectivity and limiting potential for ERCO2 compared to the CHE model. This suggests caution when using the CHE model for reactions involving multiple proton–electron transfer steps. The CEP model, which accounts for the applied potential's effect on reaction intermediates and non-redox steps, is essential for understanding the reaction mechanism and quantifying the limiting potential. ( Z. Masood and Q. Ge, Phys. Chem. C 2023, 127, 48, 23170–23179)