2025 AIChE Annual Meeting
(382ac) Electrochemical Polarization during Bimetallic Thermocatalytic Reactions
Authors
-
Synthesis and evaluation of electro-, thermo-, and photo-catalysts for applications in energy, thermochemical, and chemical manufacturing.
-
Analysis of chemical mixtures using various analytical tools.
-
Electrochemical/electrolysis deposition of thin films for semiconductor applications.
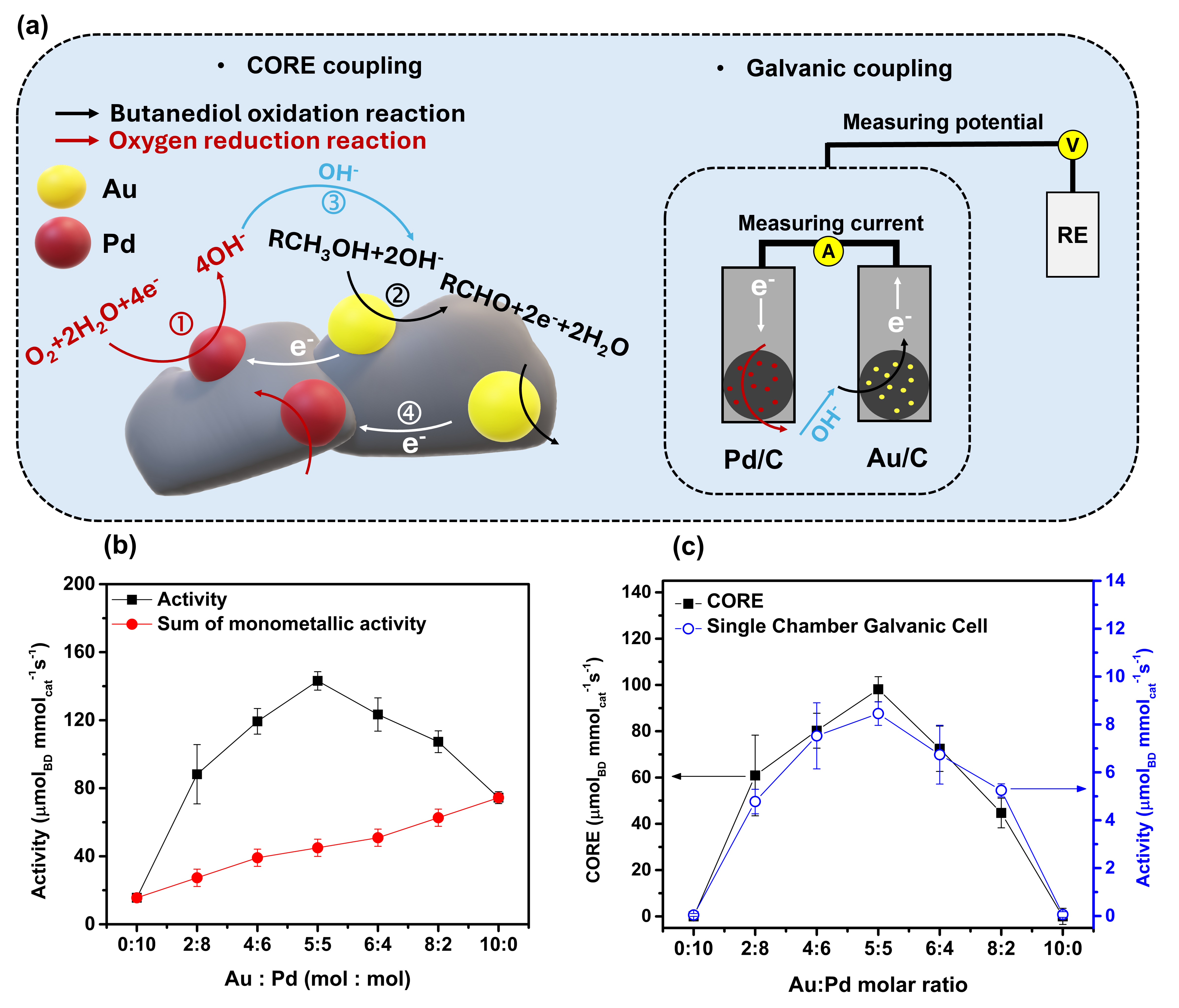
In this work, we investigate how electrochemical driving forces, specifically electrical potential, can be applied to enhance thermocatalytic rates in bimetallic catalysis without the need for external bias, using aerobic oxidation of alcohols as a model reaction. As the chemical industry shifts toward sustainability, there is a growing demand for the efficient integration of renewable energy into catalytic processes. Electrocatalysis, with its inherent ability to harness electrical energy, offers a promising route to improve conventional thermocatalytic systems.
Recent studies have shown that electrochemical principles can provide valuable insights into thermocatalytic behavior, enabling better catalyst design and operational strategies. Building on this, we explore approaches for interpreting and enhancing thermocatalytic reactions. Electrochemical techniques, such as linear sweep voltammetry (LSV) and Tafel analysis, were employed to estimate thermocatalytic reaction rates using a short-circuit model based on mixed potential theory.
By introducing a physical mixture of bimetallic catalysts, we observed spontaneous polarization between the two metals during thermocatalytic turnover, which significantly enhanced the catalytic rate, a phenomenon we refer to as Cooperative Redox Enhancement (CORE). Short-circuit experiments, where anodic and cathodic catalysts are electrically connected under thermocatalytically active conditions in a single-chamber cell, revealed that the potential difference between the physically separated catalysts generates a spontaneous current, arising from electrochemical polarization and providing direct evidence of galvanic coupling between the catalysts. Moreover, the magnitude of the current was found to correlate with the catalytic rate, allowing accurate prediction of the optimal molar ratio in the bimetallic system.
In summary, our study establishes the CORE mechanism, and introduces accessible methodologies to evaluate and design mono- and bimetallic catalysts for thermocatalytic systems, deepening our understanding of electrochemical influences on thermocatalysis and opening new pathways for developing more efficient and robust catalytic processes.