2025 AIChE Annual Meeting
(384n) Electrochemical Interface Dynamics in Microfluidic Channels: From Fabrication to Dendritic Growth
Authors
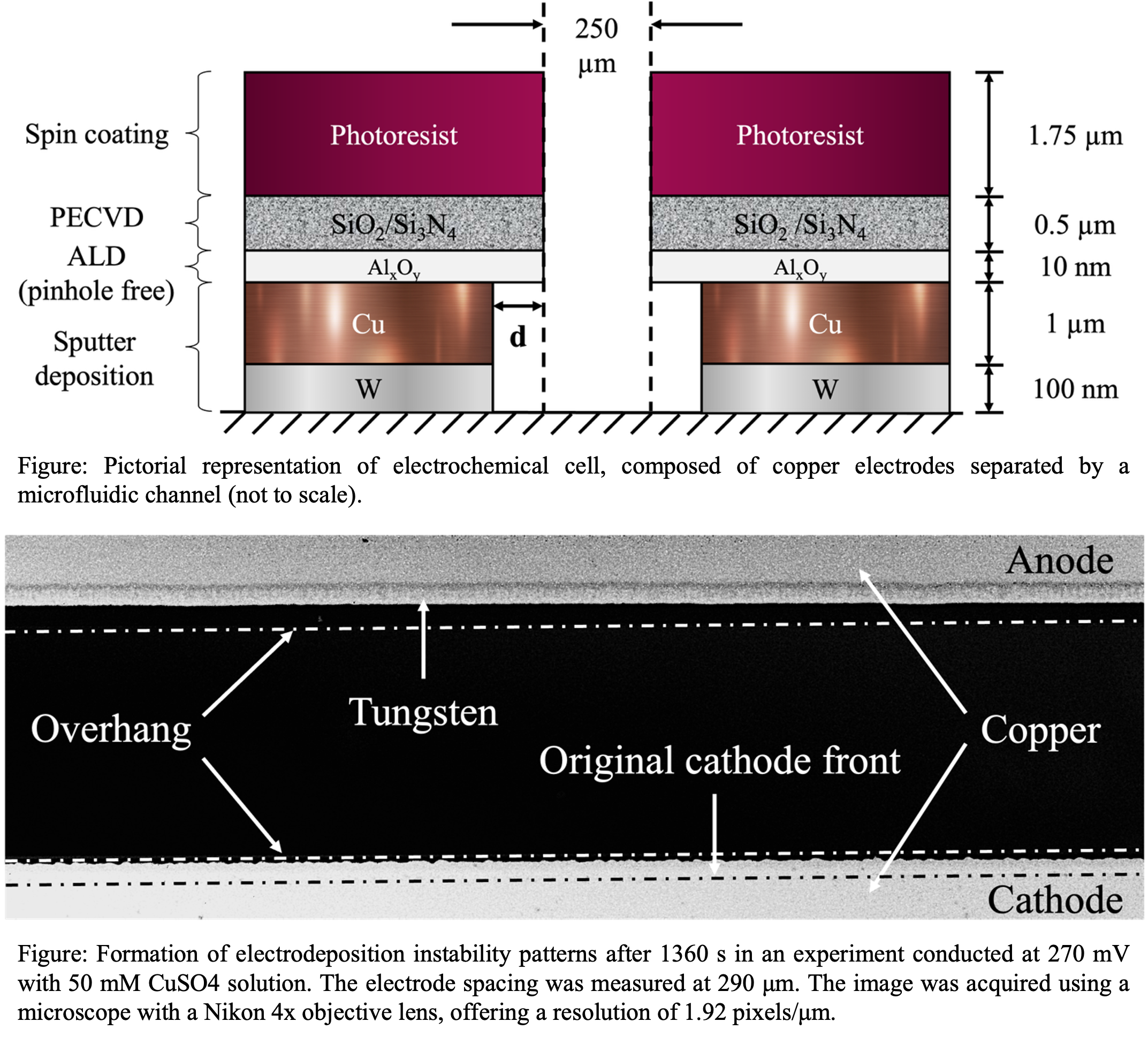
Microfluidic systems are known to offer precise control over input parameters and provide a fast response with minimum sample volumes. Hence, conducting electrodeposition with quasi-1D electrodes separated by a microfluidic channel helped in controlled growth of copper dendrites, which has significant implications for next-generation electronics and energy storage applications. In this work, we demonstrate a modified methodology to fabricate electrochemical cells that achieved quasi one-dimensional copper dendrite growth under low applied voltages. The electrodes were patterned on glass wafers through multi-step process involving sputter deposition of metal layer, dielectric film growth via atomic layer deposition (ALD) and plasma-enhanced chemical vapor deposition (PECVD), followed by photolithography and selective etching processes. The electrochemical cell consisted of copper electrodes separated by a microfluidic channel filled with a dilute copper sulfate solution. Application of a small voltage across the electrodes oxidized the copper metal to form ions at the anode, which migrate through the electrolyte and reduced at the cathode, resulting in copper metal deposition. Initially, cellular and wave-like structures appeared at the cathode, which gradually evolved into finger-like, mushroom-shaped, or branched dendritic forms. These patterns exhibited a periodic structure with superimposed wavelengths, that were identified through FFT-based image analysis. This phenomenon is known as electrodeposition — a competition between the destabilizing effects of copper ion diffusion, driven by concentration gradients and the applied electric field, and the stabilizing influence of interfacial energy between the deposited copper and the surrounding electrolyte. This platform opens new avenues for controlled pattern formation in microscale electrochemical systems, with potential applications in neuromorphic computing, energy storage, and microelectronics.