2025 AIChE Annual Meeting
(712b) Electrochemical Chloride Intercalation and Hydrogen Evolution in Membrane-Free Flow-through Reactors for Marine Carbon Dioxide Removal
Bi anodes were electrodeposited onto carbon cloth substrates using potentiostatic methods, with ongoing optimization to improve adhesion and minimize mass loss during extended operation. Cyclic voltammetry confirmed chloride intercalation peaks at −0.15 V vs. SHE, consistent with peaks associated with conversion from Bi to BiOCl (Zhang et al., 2020). NiCo cathodes, fabricated via galvanostatic deposition on carbon cloth, demonstrated HER activity with low overpotentials. Initial testing revealed operational challenges such as bubble formation, electrode degradation, and relatively high energy requirements, prompting further refinement of electrode materials and reactor design.
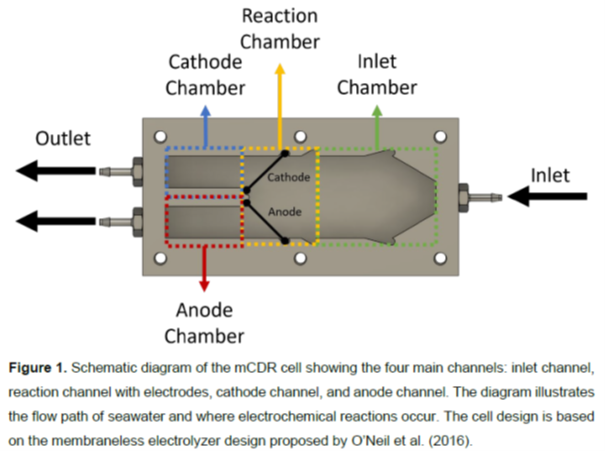
The modular V-channel reactor features angled electrodes (45°) to enhance flow dynamics and gas management while generating pH gradients critical for DIC removal (Figure 1). Preliminary experiments demonstrated single-pass DIC removal efficiency under various operating conditions, though achieving practical energy targets remains a key focus. Early-stage results highlight the feasibility of integrating chloride intercalation chemistry and HER catalysis into a scalable membrane-free system.
Future work will focus on reducing operating voltages, improving electrode durability, and validating CO₂ removal efficiency under marine-relevant conditions. This approach demonstrates potential for scalable deployment in coastal infrastructure, contributing to DOE Carbon Negative Shot goals for ocean-based CO₂ removal technologies.
References: