2025 AIChE Annual Meeting
(173d) Electrochemical Carbon Capture at Liquid/Liquid Interfaces
Addressing the critical need for clean energy production, an electrochemical carbon capture (ECC) strategy utilizing the interface between two immiscible electrolyte solutions (ITIES) is proposed to address CO2 emission reduction challenges. This approach overcomes traditional ECC limitations, such as issues with ion-selective membranes, electrode corrosion, oxygen interference, and poor solubility of redox-active compounds, by employing proton-coupled electron transfer (PCET) and facilitated ion transfer (FIT) mechanisms. PCET enables efficient CO2 desorption via simultaneous electron and proton transfer across the liquid-liquid interface, using ferrocyanide/ferricyanide and hydroquinone/benzoquinone systems. FIT enhances proton transfer efficiency through organic molecules like quinidine, significantly improving system performance. Experimental techniques such as cyclic voltammetry and Scanning Ion Conductance Microscopy analyze kinetics by miniaturizing the system and eliminating diffusion limitations (Figure 1). A theoretical model incorporating CO2 speciation, electrochemical thermodynamics, and ITIES kinetics is developed and validated experimentally, demonstrating the influence of applied potential on proton concentration kinetics at the interface. The role of quinidine in facilitating proton transport and complex pH-dependent processes is investigated. A comprehensive simulation of the carbon capture cycle identifies critical design parameters affecting CO2 absorption/desorption efficiency and energy requirements. By overcoming most of the ECC issues, especially the costly ion-selective membranes, ITIES-based system provides a promising pathway for next-generation ECC technologies, advancing fundamental understanding of liquid-liquid electrochemical dynamics and offering strategic insights for carbon capture innovation.
Figure 1: The nano-scale system (top) employs Scanning Electrochemical Microscopy (SECM) to analyze proton transfer kinetics at the ITIES using a temperature-controlled organic phase and a nanoscopic pipette tip. The macro-scale system (middle) utilizes a potentiostat and pH probe to study liquid-liquid interface dynamics with platinum working electrodes and Ag/AgCl reference electrodes. The bench-scale system (bottom) demonstrates the full ECC cycle, including CO2 absorption, electrochemical acidification and alkalinization via FIT or PCET mechanisms, and CO2 desorption in a flash tank. This multi-scale approach enables comprehensive analysis of CO2 capture efficiency and system design optimization.
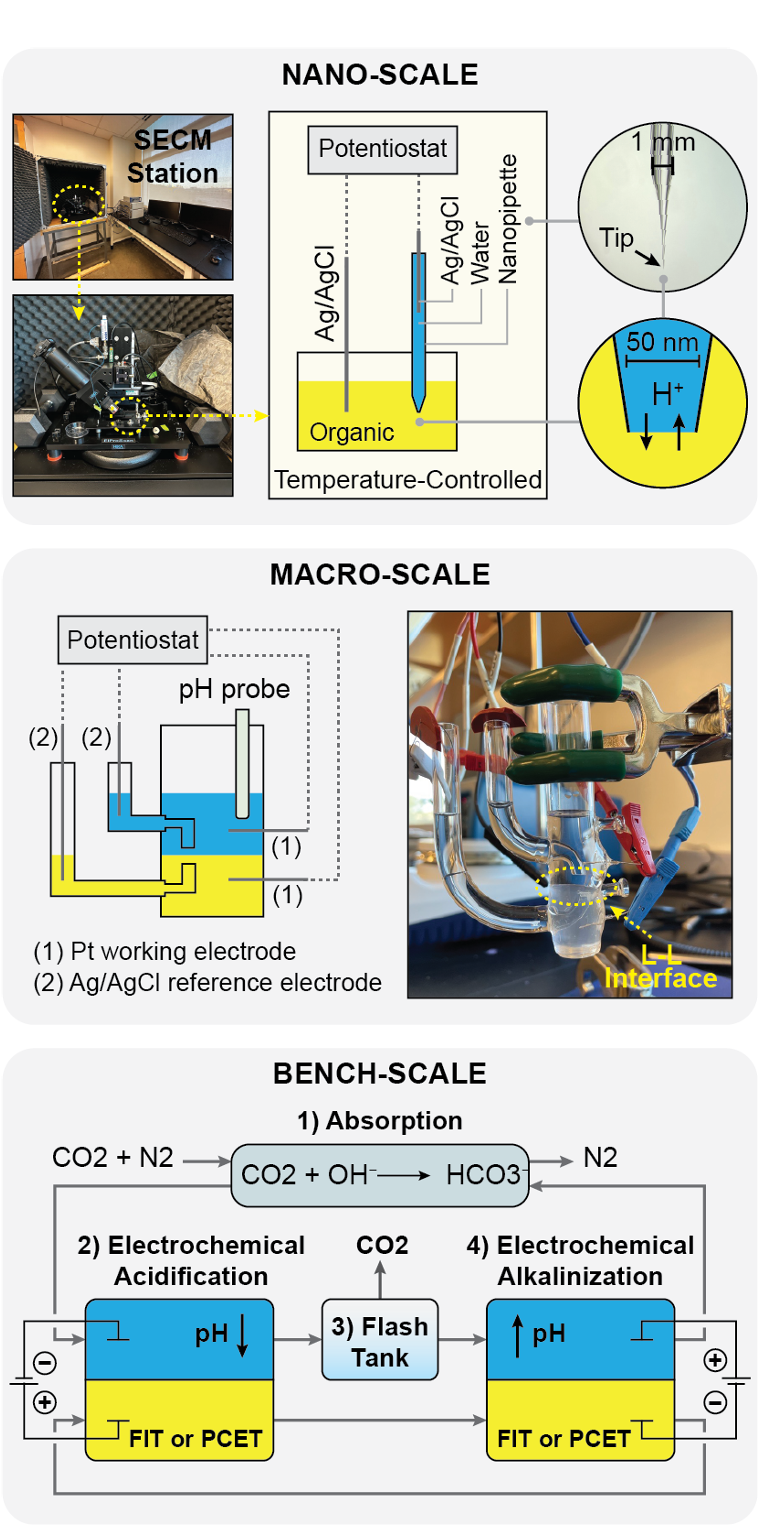
Figure 1: The nano-scale system (top) employs Scanning Electrochemical Microscopy (SECM) to analyze proton transfer kinetics at the ITIES using a temperature-controlled organic phase and a nanoscopic pipette tip. The macro-scale system (middle) utilizes a potentiostat and pH probe to study liquid-liquid interface dynamics with platinum working electrodes and Ag/AgCl reference electrodes. The bench-scale system (bottom) demonstrates the full ECC cycle, including CO2 absorption, electrochemical acidification and alkalinization via FIT or PCET mechanisms, and CO2 desorption in a flash tank. This multi-scale approach enables comprehensive analysis of CO2 capture efficiency and system design optimization.

