2025 AIChE Annual Meeting
(523c) Electrocatalytic Kinetic Insight from Thermocatalytic Gluconic Acid Oxidation on Pt Nanoparticles
Authors
Minju Chung, Massachusetts Institute of Technology
Nathan Saliceti, Georgia Tech
Karl O. Albrecht, Archer Daniels Midland
Joshua M. Terrian, ADM
David W. Flaherty, University of Illinois At Urbana-Champaign
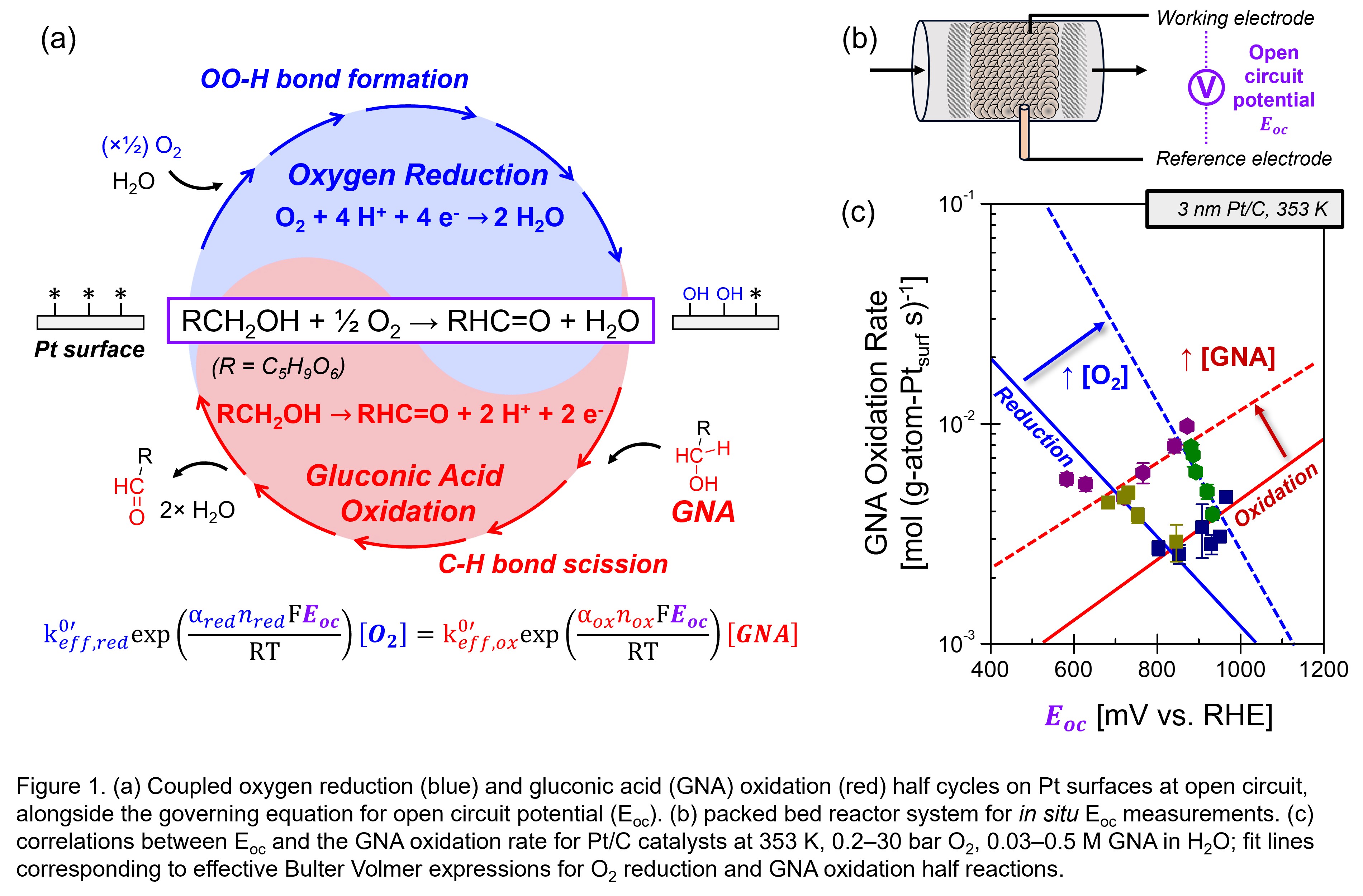
Thermochemical aerobic oxidation reactions can be conceptualized as kinetically coupled O2 reduction and substrate oxidation electrochemical half reactions (Figure 1a) where the reduction and oxidation currents are equal and the catalyst potential equals the open circuit potential (Eoc).[1] With this conceptual framework, thermochemical rates and selectivities are predictable from independent electrochemical measurements,[2] however, these analyses are limited at elevated temperatures and in packed bed reactors, especially for complex substrates such as gluconic acid (GNA, C6H12O7). Here, we bridge this gap by introducing working and reference electrodes to a packed bed reactor for in situ measurements of Eoc (Figure 1b), demonstrated for the selective oxidation of GNA to guluronic acid on Pt/C catalysts. This system accurately reaches the O2 reduction equilibrium potentials (1200–1250 mV vs. RHE, 0.2–30 bar O2, 353 K) in the absence of GNA. Kinetic dependencies give low reaction orders for GNA (0–0.3) and O2 (0–0.2), corresponding to high surface coverages of both intermediates. The corresponding Eoc values scale as a single-valued function of the O2-to-GNA ratio, with a Tafel-like slope of 120 (±20) mV per decade, arising from the additional electron required to evolve GNA C-H scission transition states relative to O2 O-H formation transition states. Furthermore, Eoc prescribes the GNA oxidation rates (Figure 1c) over a wide range of GNA concentrations (0.03–0.5 M) and O2 pressures (0.2–30 bar), where fixing the GNA concentration gives the effective Butler Volmer kinetic expression for O2 reduction, accounting for site coverages, and vice versa. Taken together, these findings reveal the inherent electrochemical half reactions embedded in aqueous aerobic oxidation catalysis.
[1] J. Ryu et. al., Nat. Catal. 2021, 4, 742.
[2] J. S. Adams, M. L. Kromer, J. Rodríguez-López, D. W. Flaherty, J. Am. Chem. Soc. 2021, 143, 7940.