2025 AIChE Annual Meeting
(538f) Effective Removal of Arsenic from Water: From Computer-Aided Design to Experimental Verification
Authors
Arsenic contamination in water significantly threatens human health and the environment. This contamination primarily results from human activities such as agricultural runoff, mining, and industrial discharge. Exposure to arsenic is associated with various health risks, including cancer, skin lesions, and neurological effects. Several techniques exist for removing arsenic from water, including chemical precipitation, coagulation-flocculation, adsorption, ion exchange, and electrochemical treatment. However, many of these methods suffer from high operational costs and limited effectiveness. Adsorption techniques have garnered attention due to low operational costs, selectivity, and high efficiency. While various adsorbent materials are available, such as metal oxides, natural clays, and activated carbon, natural clays often contain impurities that reduce arsenic removal efficiency. Therefore, the design and synthesis of synthetic clays are crucial for effective arsenic removal from water.
Design and Synthesis of Novel Adsorbents:
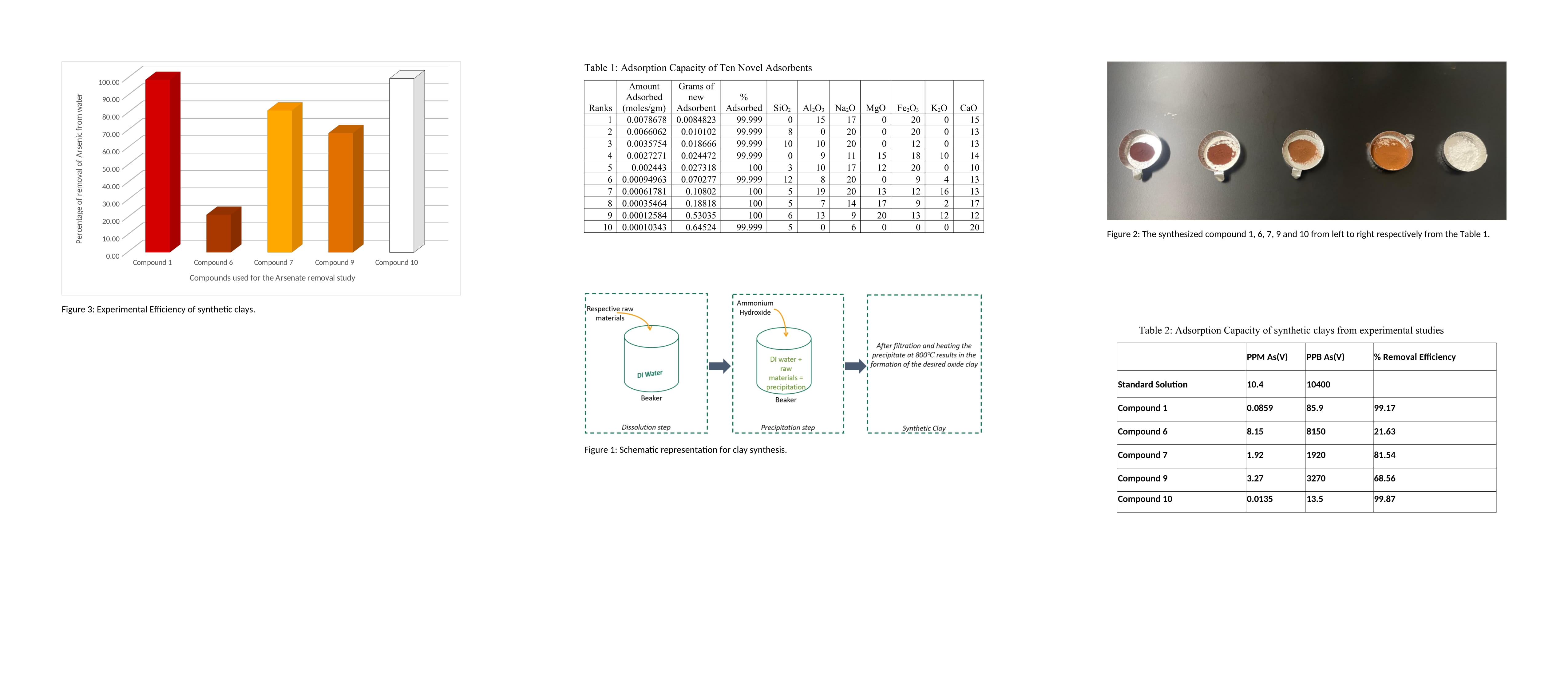
This study employs a novel approach: Computer-Aided Molecular Design (CAMD) to develop new clay-based adsorbents for arsenic removal. CAMD utilizes a reverse application of the Group Contribution Method (GCM), specifically a modified UNIFAC method, coupled with our group's novel combinatorial optimization method. The top ten predicted adsorbents and their theoretical capacities are presented in Table 1. For synthesis (Figure 1), clays were prepared based on the structures generated by CAMD (Table 1) using precipitation and thermal treatment. The synthesized clays were characterized using X-ray Fluorescence (XRF), X-ray Diffraction (XRD), and Scanning Electron Microscopy-Energy Dispersive X-ray Spectroscopy (SEM-EDS). These synthetic clays were then used to remove arsenic from contaminated water, and inductively coupled plasma mass spectrometry (ICP-MS) was used to determine the residual arsenic concentration.
Results:
The synthetic clays developed using the CAMD model (Figure 2) demonstrated significant potential for large-scale application, achieving arsenic removal efficiencies exceeding 95% at an initial arsenic concentration of 10,000 ppb. The efficacy of these adsorbents was experimentally validated at this initial concentration (Table 2 and Figure 3). The results indicate that the optimized adsorbents exhibited higher adsorption capacities than commercially available alternatives and achieved superior arsenic removal efficiencies. Future experimental studies will investigate the effects of varying pH levels, initial arsenic concentrations, and contact times.
Conclusion:
This study demonstrates the successful development of high-performance adsorbents for arsenic removal by integrating computational modeling and experimental methods. Combining CAMD with advanced material synthesis techniques offers a promising and sustainable approach to enhancing adsorption-based water treatment technologies for addressing arsenic contamination.