2025 AIChE Annual Meeting
(660e) Effect of Protonating Phosphoric Acid-Based Dopants on the Electroconductive and Mechanical Properties of PANI/Paampsa Complexes
Authors
Colton Duprey, Materials Engineering And Nanosensor (MEAN) Laboratory, Department of Chemical and Biological Engineering, The University of Alabama
Evan Wujcik, The University of Alabama
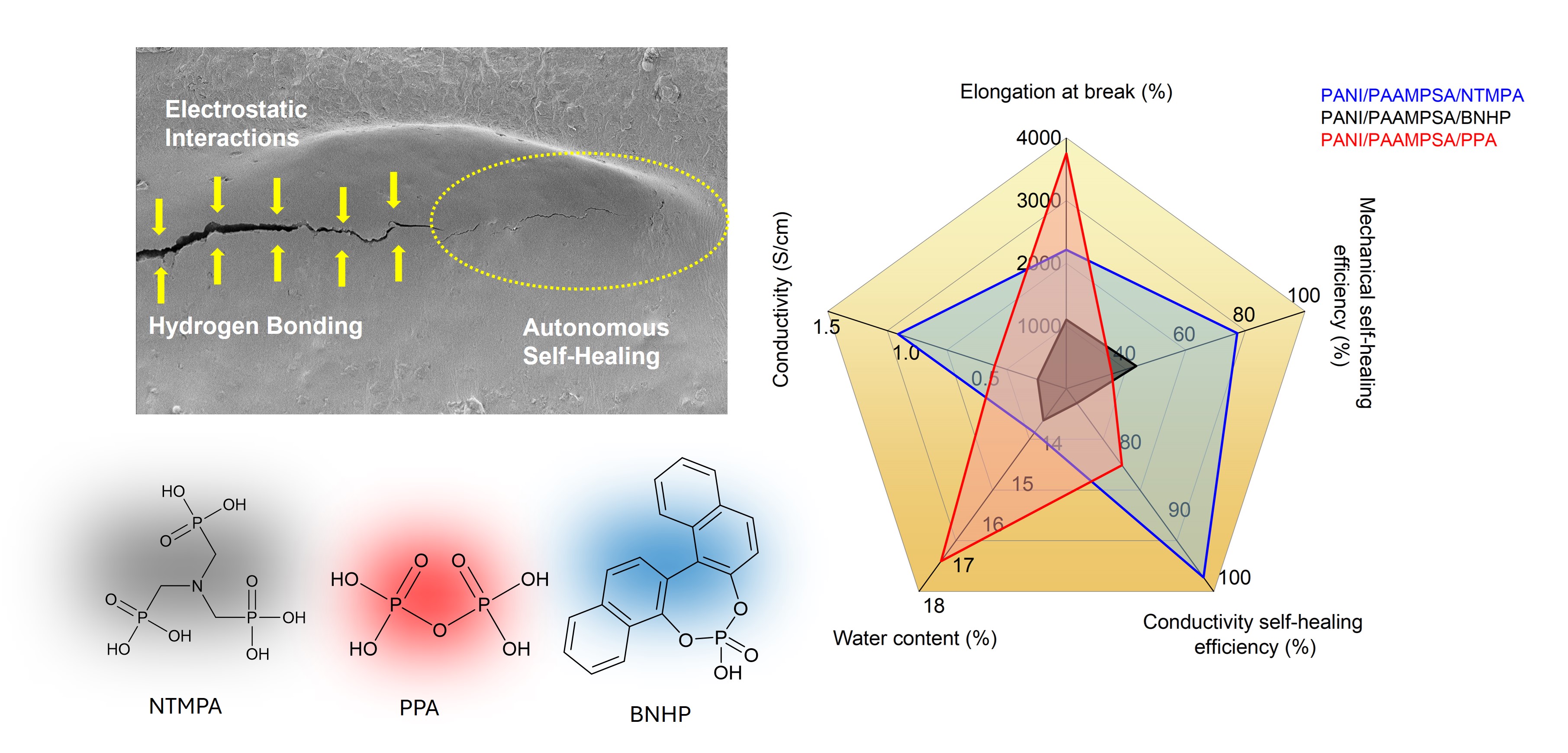
This study aims to investigate the effects of using different small molecule dopants based on phosphoric acid such as nitrilotrimethylphosphonic (NTMPA), 1,1’-binaphthyl-2,2’-diyl hydrogenphosphate (BNHP) and pyrophosphoric acid (PPA) in a polyaniline/poly(2-acrylamido-2-methylpropane sulfonic acid (PANI/PAAMPSA) polymer complex. The impact of size, structure, and number of acid groups of these small molecule dopants on the resulting solvent-casted film properties are examined. The polymer film is synthesized through a templated oxidative polymerization of aniline, where the resulting complex is non-covalently bonded via hydrogen bonding and electrostatic interactions. Fourier transform infrared spectroscopy confirmed the presence of a greater degree of hydrogen bonding in the PANI/PAAMPSA/PPA film, resulting in greater elongation at break (ϵ =3750%) and a higher percent water content (17.2% w/w). The stronger hydrogen bonding in PANI/PAAMPSA/PPA created a robust cross-linking within the material which reduced the material’s ability to self-heal. On the other hand, PANI/PAAMPSA/NTMPA film achieved high self-healing efficiencies of 98% (conductive self-healability) and 77.3% (mechanical self-healability) due to its more dynamic hydrogen bonding and electrostatic interactions. Furthermore, PANI/PAAMPSA/BNHP has shown less hydrogen bonding, stretchability, and self-healing capabilities due to the steric hindrance caused by its rigid bulky structure compared to the more linear structure of other dopants investigated.