2025 AIChE Annual Meeting
(458d) Effect of Ions on the Aqueous-Phase Adsorption of Organics on Silver
Authors
Gyan Sharma, University of Michigan
Bolton Tran, Pennsylvania State University
Nirala Singh, University of Michigan
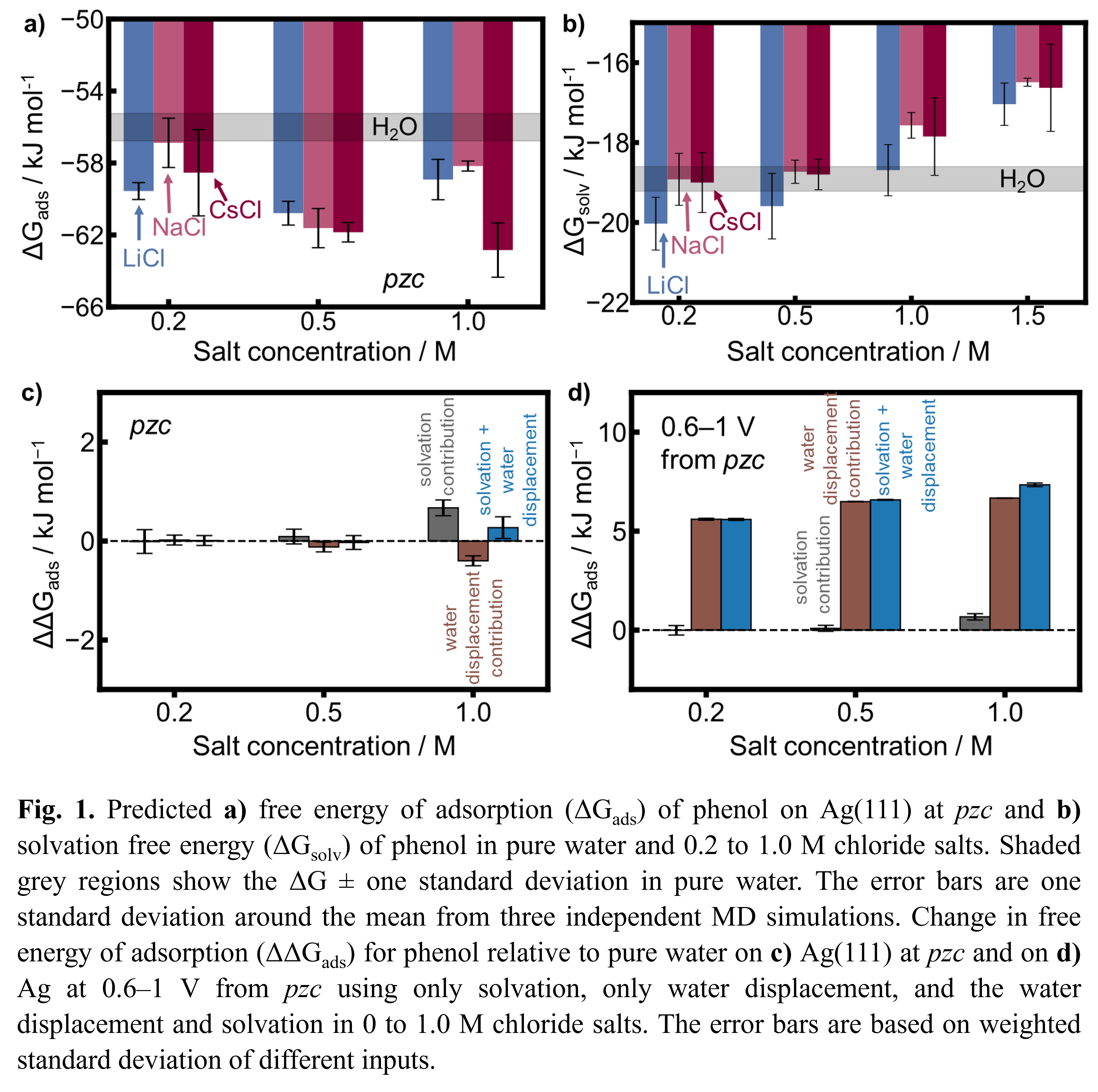
The aqueous phase adsorption of organics to surfaces is often much weaker than the gas phase due to organic solvation and the energy required for water displacement from the surface upon organic adsorption. Ions in solution can change the free energy of adsorption (ΔGads) of organics by affecting these two factors, but it is unclear which factor is most affected and by how much. We perform molecular dynamics (MD) to study the effect of 0.2 to 1.0 M LiCl, NaCl, CsCl, and NaClO4 on the solvation free energy (ΔGsolv) of phenol (Fig. 1b), catechol, benzene, guaiacol, and benzyl alcohol and their on Ag(111) at the potential of zero charge (pzc) and 298 K (for phenol in Fig. 1a), as well as the solution adhesion energy (ΔGadh). We compare the changes in the simulated ΔGads to what we would predict based on changes in ΔGsolv and ΔGadh. At the pzc, MD simulations predict that ions have a minor impact, strengthening by an average of −3.6 kJ mol−1. By temperature-dependent simulations we find these changes are roughly equally from enthalpy and entropy contributions. Based on ΔGsolv and ΔGadh changes, we predict ΔGads to only change by ±2 kJ mol−1 (for phenol in Fig. 1c) with the solvation and solution adhesion having similar impact. However, from experimental contact angle measurements on silver where the potential is more positive than the pzc and thus there is an electric field, we predict ΔGadh is much stronger than at the pzc (for phenol in Fig. 1d). While the ion effects on are small at pzc, the ion effects at potentials away from pzc are larger due to the increased effect from ΔGadh.