2025 AIChE Annual Meeting
(537e) Effect of Calcite Slit-Pore Height Variation on Methane Hydrate Dissociation/Growth Under Confinement Via MD Simulations
Authors
In the experiments by Uchida et al. [1], the largest shift in dissociation temperature (T3) for methane hydrate was noted to be -12.3 K ± 0.2 K for a 4 nm pore compared to the bulk, while for a 100 nm pore, the change in T3 was -0.5 K. In the simulation study carried out by Zhang and co-workers [3], it was found that with a decrease in slit size, hydrate melting point also goes down compared to bulk phase melting point and the melting point of methane hydrate is not affected when slit size is greater than 9 nm. Apart from pore size, the nature of porous media also affects the behaviour of the fluid within, for example, in microsecond simulations by He et al. [4] on the carbon dioxide hydrate formation showed the formation of flat carbon dioxide nanobubble on the hydrophobic graphite surface; while the partial cylindrical nanobubble of carbon dioxide is formed with the hydrophilic silica surface.
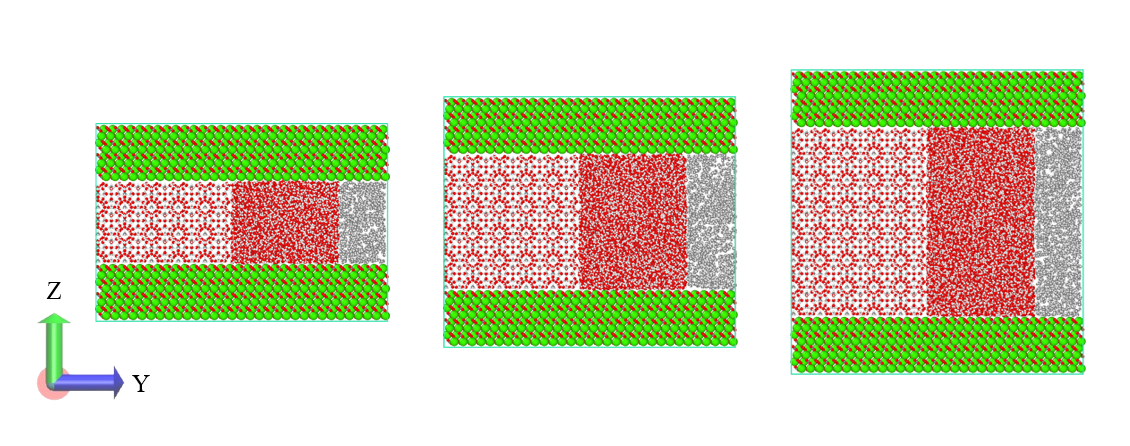
Although silica slit-pores are investigated more extensively in MD simulations, hydrate reservoirs are also made of carbonates, which might have a different influence than silicates. Therefore, in this work, we are studying the effect of pore height variation for calcite slit-pore on the T3 of methane hydrate, along with the behaviour of confined fluids inside calcite pores. For that purpose, we have selected three different slit-pore heights of ~3.8, 6.2, and 8.6 nm. We have used the open-source package GROMACS 2024 to perform the MD simulations. The temperature values selected to perform these non-equilibrium MD simulations in NVT ensembles are 280, 290, and 300 K. The choice of these temperature values is based on the T3 determination for methane hydrate through the TIP4P/Ice model by Conde and Vega [5] for pressure values of 40, 100, and 400 bar. Preliminary results show that, unlike the hydrophilic silica slit-pore studied previously in our group [6], the methane nanobubble formed inside the calcite slit-pore prefers to stay away from the calcite surface. This difference in the behavior of methane nanobubble under calcite confinement, may be due to the surface interaction leading to the formation of a bound water layer as a result of the strong electrostatic interactions between water molecules and calcite surface atoms. Further, we plan to look into the arrangement of bound water molecules near the calcite surface and its influence on the hydrate structure close to the surface, as well as on the methane nanobubble. For the lower temperature simulation, where methane hydrate growth is expected, cage identification algorithms will also be employed to identify the hydrate structure. Apart from this, we plan to investigate the change in the dissociation temperature (T3) of methane hydrate across all three different pore heights and find the correlation of T3 with respect to pore heights.
References:
[1] T. Uchida, T. Ebinuma, S. Takeya, J. Nagao, H. Narita, Effects of pore sizes on dissociation temperatures and pressures of methane, carbon dioxide, and propane hydrates in porous media, J. Phys. Chem. B 106 (2002) 820–826. https://pubs.acs.org/doi/10.1021/jp012823w.
[2] Y. Qin, Z. Pan, Z. Liu, L. Shang, L. Zhou, Influence of the Particle Size of Porous Media on the Formation of Natural Gas Hydrate: A Review, Energy Fuels 35 (2021) 11640–11664. https://doi.org/10.1021/acs.energyfuels.1c00936.
[3] Z. Zhang, P.G. Kusalik, N. Wu, C. Liu, Y. Zhang, Molecular simulation study on the stability of methane hydrate confined in slit-shaped pores, Energy 257 (2022) 124738. https://doi.org/10.1016/J.ENERGY.2022.124738.
[4] Z. He, F. Mi, F. Ning, Molecular insights into CO2 hydrate formation in the presence of hydrophilic and hydrophobic solid surfaces, Energy 234 (2021) 121260. https://doi.org/10.1016/j.energy.2021.121260.
[5] M.M. Conde, C. Vega, Determining the three-phase coexistence line in methane hydrates using computer simulations, J. Chem. Phys. 133 (2010) 64507. https://doi.org/10.1063/1.3466751/189083.
[6] B. Moorjani, J. Adhikari, S. Hait, Molecular insights into methane hydrate dissociation under confinement in a hydrophilic silica nanopore, Fluid Phase Equilib. 588 (2025) 114218. https://doi.org/10.1016/J.FLUID.2024.114218.