2025 AIChE Annual Meeting
(301a) Effect of Aqueous Solvent Composition on the Nucleation Kinetics and Crystal Habit
Authors
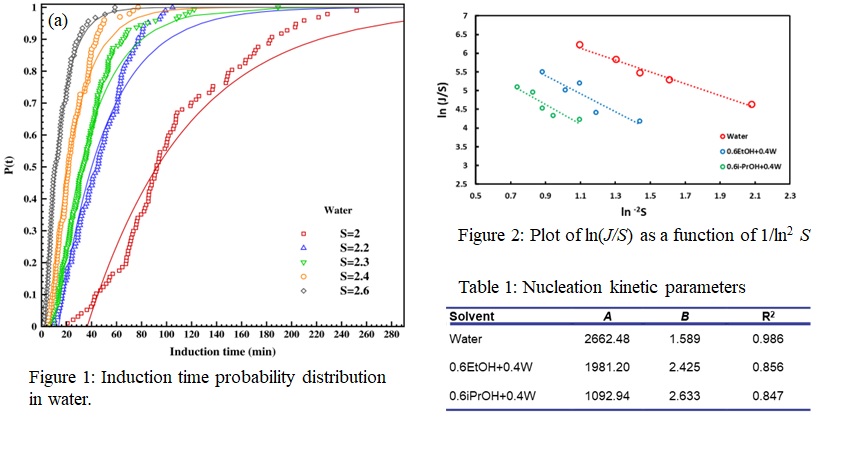
The present work investigates the influence of alcohols, specifically ethanol and isopropanol, as co-solvents in water on the nucleation kinetics of L-ascorbic acid using the isothermal method. Induction times were measured over 80 times at five different supersaturation (S) levels utilizing the Crystal16V3 instrument from Technobis Crystallization Systems. The experimentally obtained induction time data points were used to determine the probability of nucleus formation within a given time frame (Fig. 1). Nucleation kinetics were analyzed using classical nucleation theory, which establishes a relationship between nucleation rate and supersaturation, as plotted in figure2.
Additionally cooling crystallization experiments were performed using CrystallineV2 instrument to visualize the effect of solvent system on crystal habit.
With the addition of alcohol in water, the kinetic parameter (A) decreases, indicating less nucleation site whereas the thermodynamic factor (B) increases, implying a high activation energy for nucleation (Table 1). Ascorbic acid, being a polar molecule, has a high affinity with polar solvents such as water. The addition of alcohol reduces the polarity of the solvent mixture resulting in lower solubility in water-alcohol solvent systems and subsequently affecting the nucleation kinetics. Furthermore, adding alcohol in water during cooling crystallization experiments transforms the crystal habit from cubic to crystals with prominent growth along one crystallographic axis resulting in lengthened prism-shaped crystals in water-alcohol binary solvent systems.