2025 AIChE Annual Meeting
(509c) Dynamic Probe of CO2 Dissolving for CO2 Storage and Conversion Via Micro-IR Spectroscopy
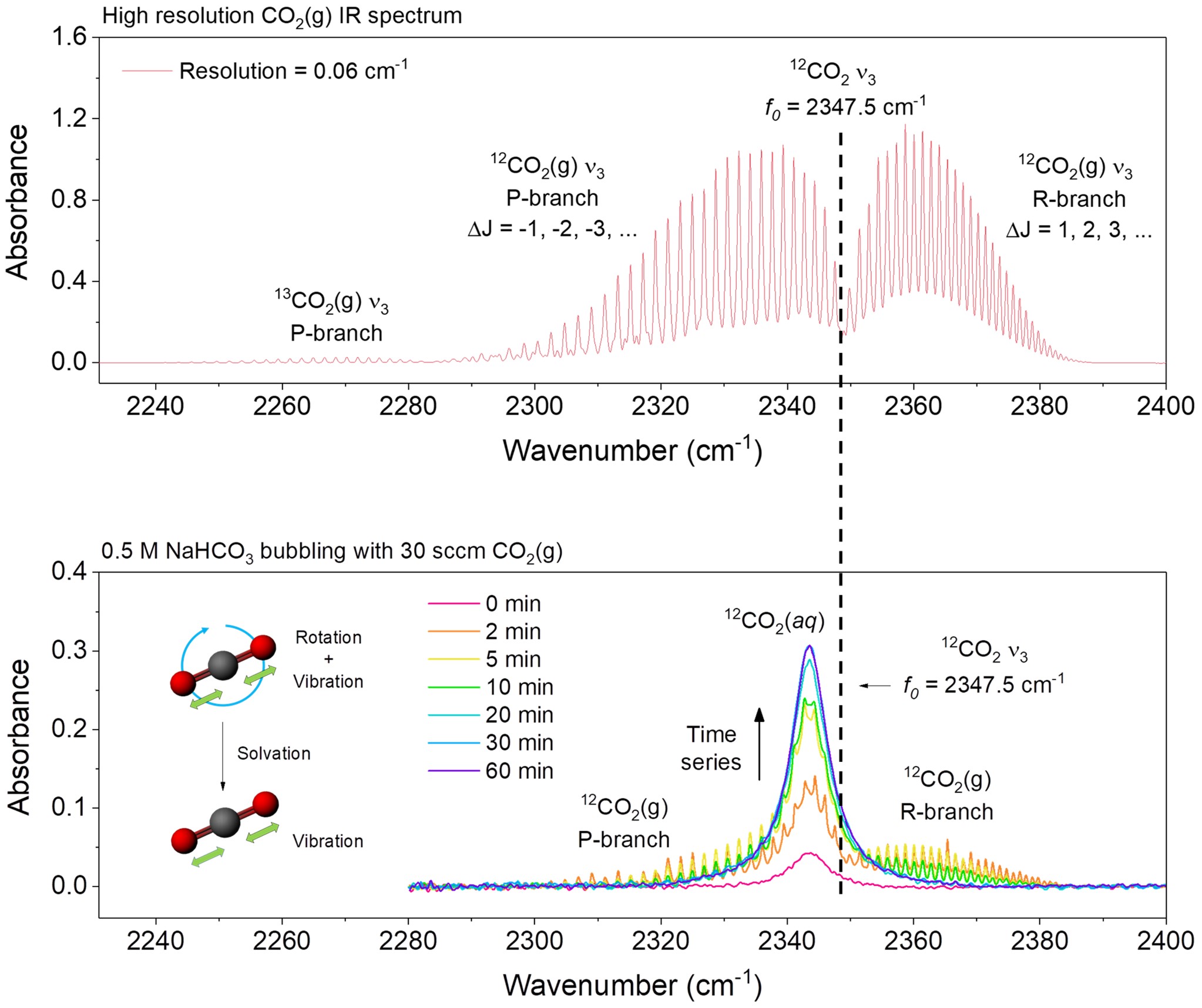
Recently, many efforts have been spent on developing novel strategies for CO2 storage and conversion to close the carbon cycle and mitigate the climate challenge. To obtain insightful understandings to these processes for further system optimization, here we develop a dynamic micro-IR spectroscopy to track and quantify the dissolving of CO2 in various aqueous solutions under a wide pressure range up to ~ 60 atm (at which point gaseous CO2 starts to liquefy). As CO2 molecules starting to interact with H2O in the solution, dynamic micro-IR tracking shows that the quantized CO2(g) rotational state transitions quench quickly while only the vibrational transition remains as an identical signal of dissolved CO2(aq), indicating the molecular mechanism of CO2 solvation to be the hindering of molecular rotation by water. The quantitative monitoring of CO2 dissolving and desolvation reveals a quicker solvation kinetics than desolvation, rationalizing the widely observed CO2 supersaturation in natural waterbodies. Employing the derived CO2 molar extinction coefficient, we corelate the CO2(aq) concentration with the Faradaic efficiency of electrocatalytic CO2 conversion to formate and observe a high linearity, indicating a clear mechanism pattern of CO2 electroreduction determined by CO2 availability. Our dynamic CO2 probing technique enables a further quantitative optimization on the design of CO2 storage and conversion systems.