2025 AIChE Annual Meeting
(523e) Disentangling Magnitudinal and Directional Contributions in Transient Degrees of Rate Control Calculations for Partial Oxidation Reactions with Cyclic Steady States
Authors
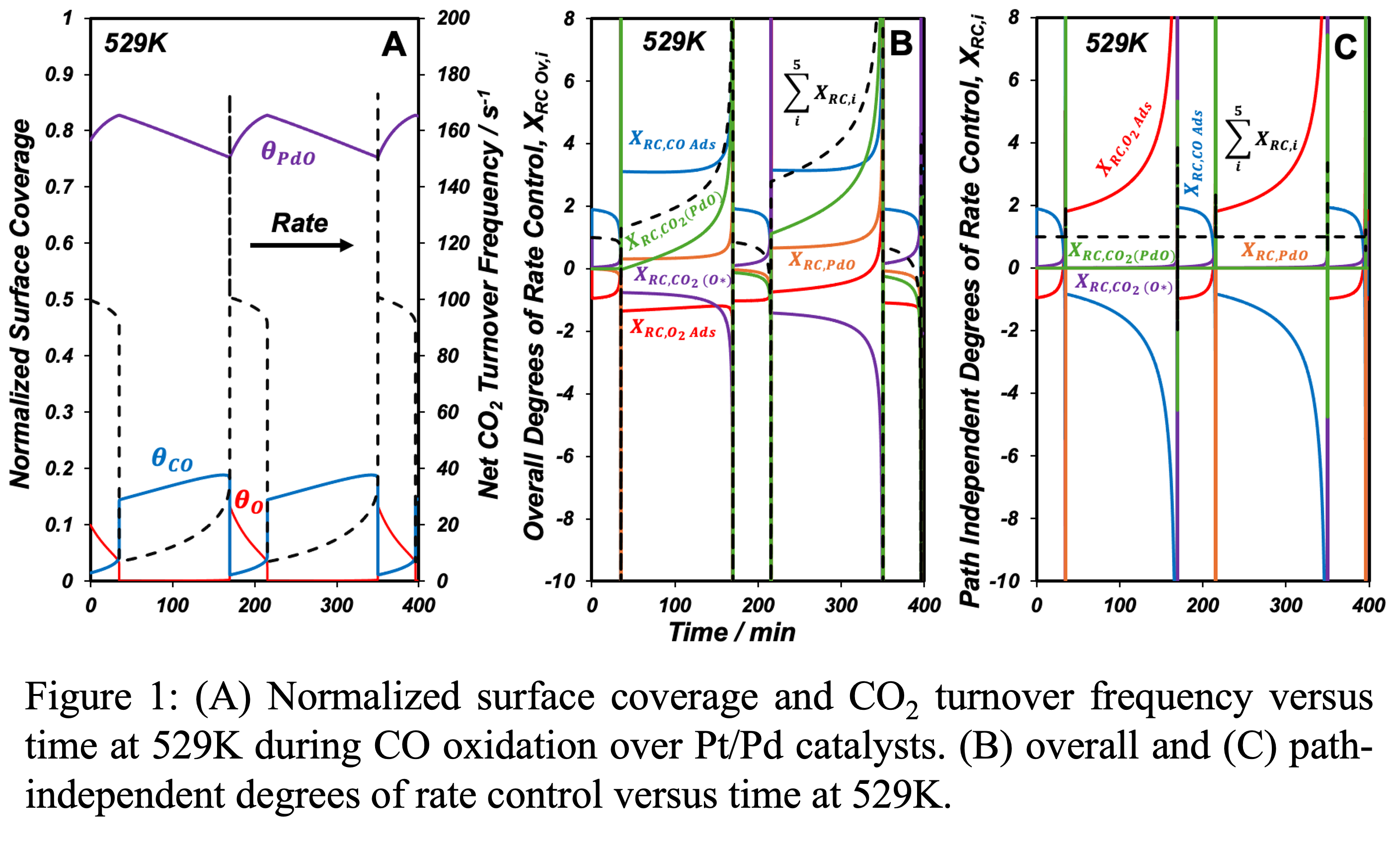
In this work, we use the special case of stable limit cycles to deconvolute path-dependent and path-independent degrees of rate control that carry the directional and magnitudinal contributions to the transient degrees of rate control (DRC). Applying the conventional DRC definition to a CO oxidation reaction system exhibiting oscillatory steady states (Figure 1A) yields uninterpretable results, as demonstrated by the calculations in Figure 1B. Figure 1C, on the other hand, plots path-independent DRCs which form regular patterns and sum to unity. Our work presents a novel, potentially generalizable formalism for assessing rate determining steps under transient conditions, and suggests paring of path-dependence (or directionality) as key to rigorously determining rate determining steps under transient conditions.
(1) Campbell, C. T. The Degree of Rate Control: A Powerful Tool for Catalysis Research. ACS Catal. 2017, 7 (4), 2770–2779.
(2) Motagamwala, A. H.; Dumesic, J. A. Microkinetic Modeling: A Tool for Rational Catalyst Design. Chem. Rev. 2021, 121 (2), 1049–1076.
(3) Foley, B. L.; Bhan, A. Degrees of Rate Control at Non(Pseudo)Steady-State Conditions. ACS Catal. 2020, 10 (4), 2556–2564.