2025 AIChE Annual Meeting
(4c) Direct Hydrogenation of CO2 to Light Olefins and Paraffins over the Synergetic ?-Fe2O3-?-Fe5C2/Al2O3 Catalyst
Thermocatalytic hydrogenation of CO2 into synthetic fuels is an attractive pathway to reduce our dependence on fossil fuels. Hydrogen can be generated via water electrolysis using renewable electricity. In this study, reverse microemulsion (RME) method was implemented to reduce the nanoparticle size in the Fe-based catalysts. In the RME system, nanosized water droplets containing reactants are surrounded by surfactant molecules and dispersed in a continuous oil phase. Nanodroplets act as microreactors, restraining the amounts of reactants and limiting the nanoparticle size.
A series of catalysts with alkali- and transition metal-promoters were synthesized. The RME synthesis resulted in a high specific surface area (ca. 200-250 m2/g) and well dispersed Al2O3-supported Fe3O4 nanoparticles (5-10 nm). Under reaction conditions Fe3O4 nanoparticles convert to a mixture of α-Fe2O3 and χ-Fe5C2 nanoparticles promoting reverse water gas shift (RWGS) and hydrogenation.
The synthesized catalysts were tested for direct, one-throughput CO2 hydrogenation to lower olefins and paraffins (C2-C6). Fresh and spent catalysts were characterized by XRD, TPR, TPD, STEM-EDS, TGA-FTIR, and XPS. The α-Fe2O3-χ-Fe5C2/Al2O3 catalysts showed excellent performance, with C2-C6 selectivity and CO2 conversion above 55% at 375 °C, 10 bar, 1000 mL/(kg h), and H2/CO2 = 4. Parameter sensitivity analysis was conducted investigating the effects of temperature, pressure, space velocity, and feed composition and optimizing the performance. Catalyst stability was also investigated showing only a minor decline over 100 h on stream.
The RME method resulted in superior catalytic performance due to the reduced inital nanoparticle size that promoted the formation of χ-Fe5C2 for hydrogenation, while also forming the α-Fe2O3 phase responsible for RWGS, resulting in a synergetic effect.
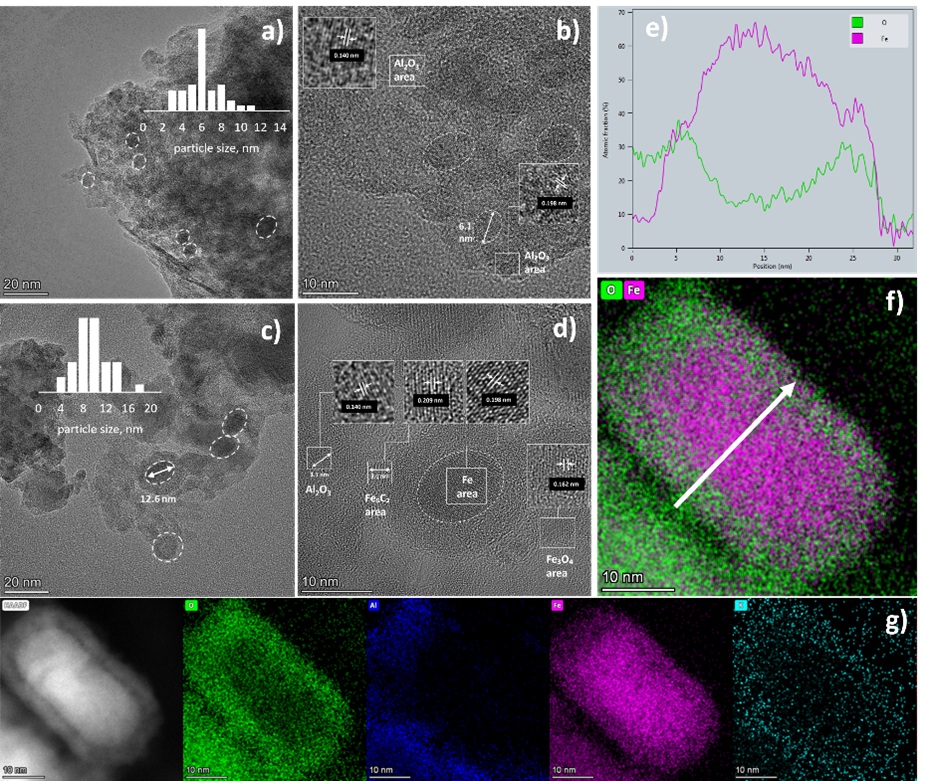
A series of catalysts with alkali- and transition metal-promoters were synthesized. The RME synthesis resulted in a high specific surface area (ca. 200-250 m2/g) and well dispersed Al2O3-supported Fe3O4 nanoparticles (5-10 nm). Under reaction conditions Fe3O4 nanoparticles convert to a mixture of α-Fe2O3 and χ-Fe5C2 nanoparticles promoting reverse water gas shift (RWGS) and hydrogenation.
The synthesized catalysts were tested for direct, one-throughput CO2 hydrogenation to lower olefins and paraffins (C2-C6). Fresh and spent catalysts were characterized by XRD, TPR, TPD, STEM-EDS, TGA-FTIR, and XPS. The α-Fe2O3-χ-Fe5C2/Al2O3 catalysts showed excellent performance, with C2-C6 selectivity and CO2 conversion above 55% at 375 °C, 10 bar, 1000 mL/(kg h), and H2/CO2 = 4. Parameter sensitivity analysis was conducted investigating the effects of temperature, pressure, space velocity, and feed composition and optimizing the performance. Catalyst stability was also investigated showing only a minor decline over 100 h on stream.
The RME method resulted in superior catalytic performance due to the reduced inital nanoparticle size that promoted the formation of χ-Fe5C2 for hydrogenation, while also forming the α-Fe2O3 phase responsible for RWGS, resulting in a synergetic effect.

