2025 AIChE Annual Meeting
(123f) Digital Twins and Physics-Informed Neural Networks (PINNs) for the Modelling and Design of Industrial Bioreactors: The Case Antivenom Nanobody Producing E. coli
Authors
The application of digital twins (Figure 2) in conjunction with classical methods depends on the availability of large volumes of data; such data are typically scarce for bioreactors. PINNs have emerged as promising means to integrate first principle-based models with data. Unlike traditional neural networks that rely purely on data, PINNs embed physical laws-typically expressed as ordinary or partial differential equations-directly into the loss function during training. This enables the model to learn not just from observations, but also from governing scientific principles, thereby improving generalization, interpretability, and performance in data-scarce environments. PINNs have been already successfully applied in diverse domains such as fluid dynamics, heat transfer, image reconstruction and parameter estimation offering a compelling framework for developing reliable digital twins in biomanufacturing by combining the strengths of data-driven modeling with mechanistic consistency. PINNs are subsequently tested in an extensive round of experiments that includes (a) batch and semi-batch bioreactor models; (b) models where the modelling expression is under scrutiny or unknown.
In the first round of experiments, we demonstrated the ability of PINNs to capture state-space dynamics and provide accurate short- and long-term state predictions for both batch and semi-batch bioreactor models (Figure 3). We begin by assessing the capacity of PINNs to perform digital twinning by learning the system’s kinetic parameter space as the process evolves. Unlike conventional methods that require full data trajectories for parameter estimation and model identification, the PINN-based approach enables continuous learning and real-time estimation of unknown kinetic parameters. This dynamic capability allows for more adaptive and responsive modeling, enhancing the overall utility of digital twins in bioprocess applications. Results are illustrated (Figure 4 and Figure 5) and show that effective digital twinning is achievable even with minimal data availability. Remarkably, PINNs were able to perform accurate system state and space estimation with as few as two data points, highlighting their robustness in data-scarce scenarios. This capability is possible because PINNs incorporate known physical laws directly into the learning process. In the semi-batch case, PINNs maintained reliable performance even when encountering discontinuous changes in state space—such as sudden variations in inlet flow rate—where traditional models often struggle. Accurate state estimation allows for timely adjustments to operating conditions based on the current state of the bioreactor, supporting proactive and informed decision-making. Furthermore, as the PINN continues to learn during operation, it dynamically explores the kinetic parameter space, moving beyond the limitations of fixed-parameter models. This adaptive modeling framework enables flexible and responsive control strategies, which are essential for maintaining optimal process conditions, mitigating risks, and improving overall biomanufacturing efficiency.
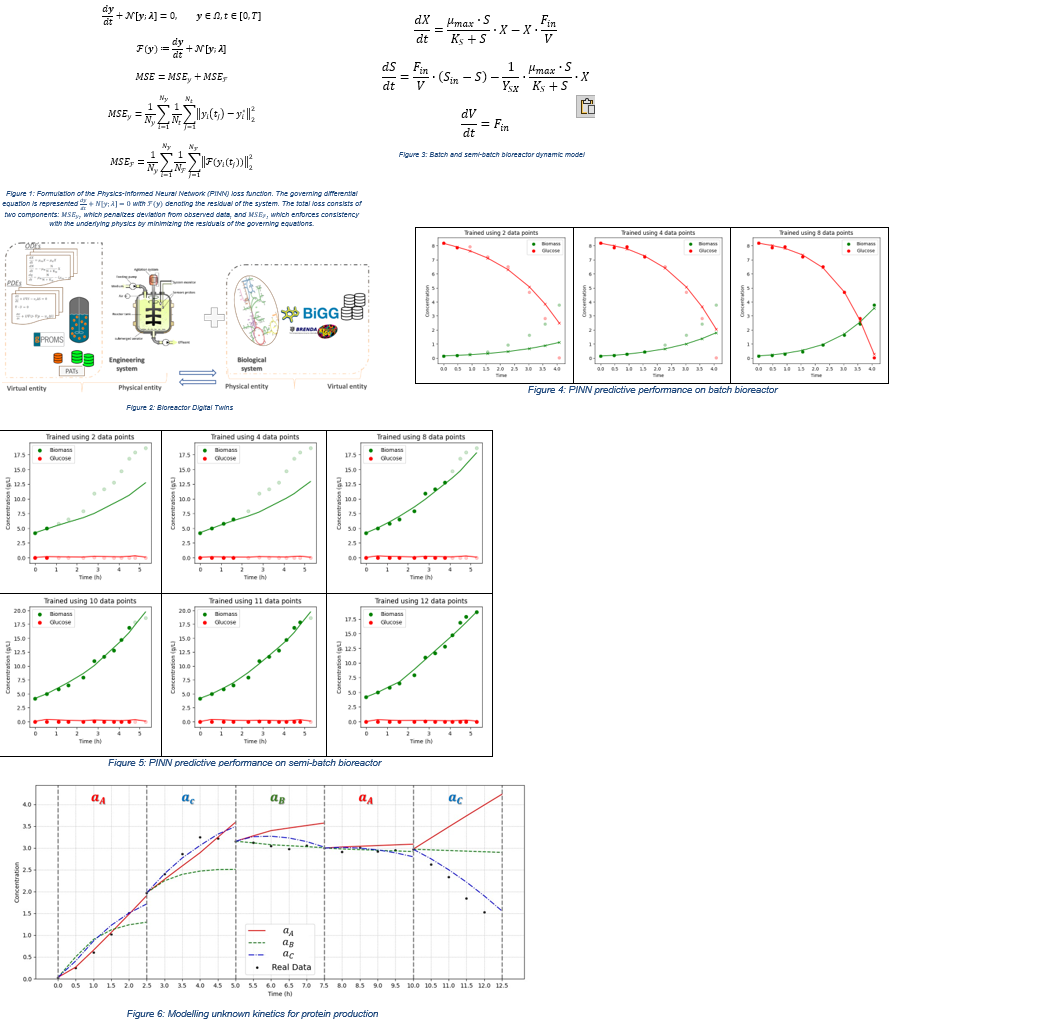
Modelling expressions for the second round relates to unknown kinetics for protein production; kinetics need to be discovered from a selected set of options that could be extensive and related to curated kinetics available from upstream analysis of the unknown dynamics of the cell. As the process evolves, the digital twin autonomously determines the most suitable kinetic model while concurrently optimizing its associated parameters. This selection is guided by a defined criterion based on the training loss of the PINN, ensuring both model accuracy and physical consistency. The results (Figure 6) demonstrate that this approach can accurately identify the most appropriate kinetic expression for the previously unknown protein production dynamics. This capability is particularly valuable in bioprocess development, where mechanistic knowledge is often incomplete or uncertain. Accurately uncovering the underlying kinetics enables more reliable process predictions, better control strategies, and faster development cycles.
This work highlights the significant potential of Physics-Informed Neural Networks as hybrid modeling tools for digital twins in industrial bioreactor applications. By integrating physical laws into the learning process, PINNs enable accurate state estimation and kinetic parameter identification even in data-scarce and dynamically changing environments. The experimental results demonstrate that PINNs outperform conventional data-driven models in both predictive accuracy and adaptability and can effectively handle unknown or evolving system dynamics. These strengths position PINNs as powerful enablers for advanced control strategies, particularly Model Predictive Control (MPC), where reliable, real-time predictions are critical for optimizing process performance, ensuring product quality, and maintaining safe and stable bioprocess operations.