2025 AIChE Annual Meeting
(158b) Digital Twin Development for Continuous Manufacturing of Xrna-Based Vaccines and Therapeutics
Authors
Busuyi Adebayo - Presenter, Missouri University of Science & Technology
Kaschif Ahmed - Presenter, Biogen
Edita Botonjic-Sehic, Arrabta Bio
Aaron Cowley, ReciBioPharm
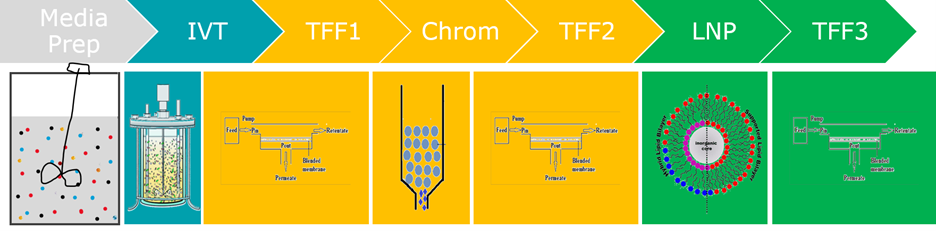
The biopharmaceutical industry is facing increasing demands for rapid, scalable, and cost-effective production of xRNA-based therapeutics and vaccines. Meeting these demands requires a shift from traditional batch manufacturing toward integrated, continuous production platforms that offer improved process control, consistency, and agility.
A major innovation in this space is the unification of upstream and downstream operations into a continuous manufacturing system. This real-time, closed-loop approach enhances product quality, reduces time-to-market, and aligns with regulatory initiatives focused on Quality by Design (QbD), Process Analytical Technology (PAT), and real-time release testing (RTRT).
Our work supports this transition by advancing two core areas:
- Mechanistic Modeling of Unit Operations – Via model-based process development, we analyze key processes such as in vitro transcription (IVT) reaction, purification, and formulation to understand how process conditions impact product quality.
- Model Validation and Data Integration – We ensure model credibility through rigorous validation with experimental data, enabling predictive and regulatory-aligned applications.
Together, these efforts lay the groundwork for digital twins capable of enabling real-time monitoring, optimization, and control of xRNA manufacturing. This approach contributes to a more responsive and resilient biomanufacturing ecosystem, ultimately accelerating the delivery of advanced therapeutics.