2025 AIChE Annual Meeting
(189x) Differential Scanning Calorimetry Experiments in Nanoconfinement: Novel Mesoporous Silica Monoliths for Understanding Confinement Effects on Fluid Thermal Behavior
Introduction
Nanoconfinement plays a crucial role in modifying the thermodynamic properties of fluids, significantly impacting phase transitions, namely, boiling points and melting behaviors. Fluids confined within nanopores experience altered intermolecular interactions due to surface effects, leading to deviations from bulk-phase behavior. Understanding these effects is essential for advancements in catalysis, nanofluidic systems, shale oil recovery, and controlled drug delivery. The Kelvin equation, which describes vapor pressure depression in confined systems, provides a theoretical framework for predicting changes in boiling and freezing points:
ln(P/P0) = -2γVm/RTr
Where P is the vapor pressure of the confined fluid, P0 is the bulk-phase vapor pressure, γ is the surface tension, Vm is the molar volume, R is the universal gas constant, T is the temperature, and r is the pore radius. This equation predicts that the fluid boiling point should decrease as pore size decreases.
This study aims to experimentally investigate the confinement effects in the thermal behavior of fluids by conducting Differential Scanning Calorimetry (DSC) experiments on fluids within novel mesoporous silica monoliths, enabling a detailed analysis of thermal behavior under nanoconfinement.
Methodology
Synthesis of Mesoporous Silica Monoliths
Mesoporous silica monoliths were synthesized using a sol-gel method with a block copolymer as a template, followed by careful drying and pyrolysis in a tube furnace under an inert nitrogen atmosphere. This results in unburned SBA 16 powder, which still has pyrolyzed carbon inside the pores and surface. The structures feature a narrow pore size distribution ranging from 3 nm to 5 nm, characterized by nitrogen adsorption-desorption isotherms for surface area and pore size distribution analysis.
The produced powder was then sintered at 2 pressure settings and high temperature. The sintered powder (monolith) was healed by the same precursor sol-gel block copolymer solution to fill the intergrain void (macropores) until only the SBA 16 nanopores were left. The monoliths were characterized once more with:
- Nitrogen adsorption-desorption isotherms for surface area and pore size distribution analysis.
- Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) for morphological assessment.
DSC Measurements
DSC experiments were conducted on bulk fluids and fluids confined within silica monoliths to compare thermal transitions. Fluids selected for this study included water and benzene — representing a range of polarities and molecular interactions. DSC scans were performed under controlled heating rates to capture boiling shifts and controlled cooling and heating to capture supercooling effects. All tests were performed under an inert nitrogen atmosphere, at either ambient or higher pressure.
Results and Discussion
Impact of Nanoconfinement on Freezing and Boiling Points
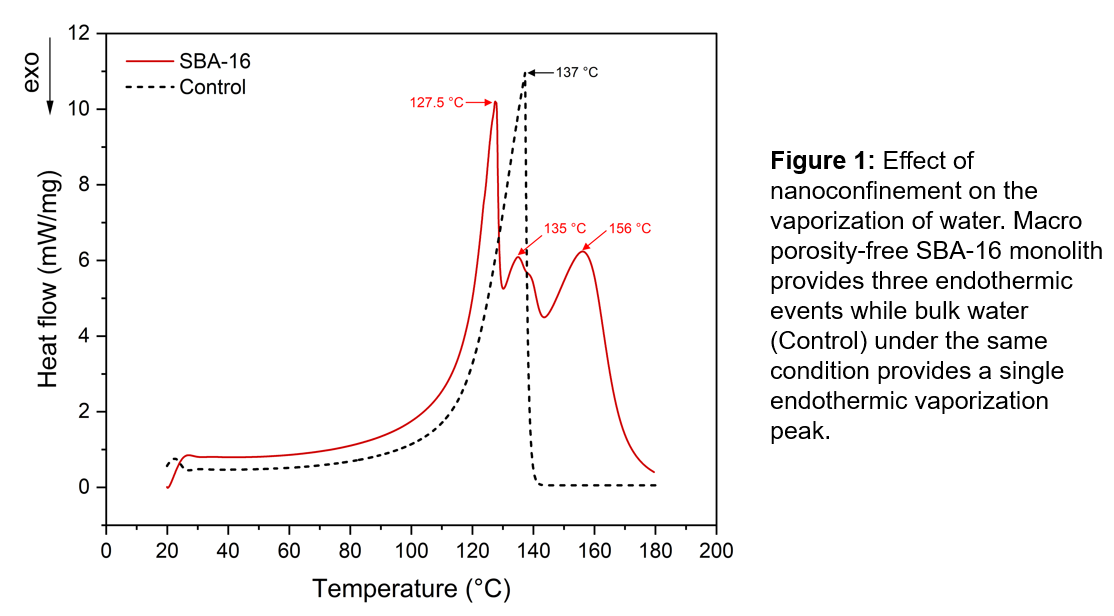
DSC results demonstrated significant alterations in freezing and melting points for confined fluids. For instance, water confined in SBA 16 monolith pores exhibited multiple boiling peaks. The first peak, at a lower temperature than its bulk counterpart, aligns with Kelvin equation predictions. However, that model is not capable of predicting the presence of the later peaks obtained. The multiple peaks have been attributed to a layer-by-layer model of phase change. Benzene showed only one peak. However, its confinement peak is not as sharp as its bulk counterpart. The singular peak of benzene over the multiple water peaks is attributed to the surface interaction between fluid molecules and the pore inner surface.
Confinement freezing effects were also observed within the pores. Water presented multiple freezing peaks as well, whereas bulk water froze at 0°C. This also suggests layer-by-layer dynamics, where confinement inhibits the formation of stable ice nuclei.
Conclusion
This study provides a detailed experimental validation of nanoconfinement effects on fluid thermodynamics. Although some boiling peaks obtained reinforce theoretical models such as the Kelvin equation, said equation cannot predict the multiple peaks that the results show. These insights have profound implications for fields ranging from thermal energy storage to advanced separation processes and drug delivery, where phase change behavior under confinement dictates performance. Future work will extend these studies to modifying the inner surface chemistry of the pores and DSC experiments under a variety of pressure conditions, further unraveling the complexities of confined phase transitions.