2025 AIChE Annual Meeting
(563d) Development of a Pressure Swing Adsorption Reactor for the Production of NH3
Authors
One way to intensify a process is to carry out reaction and separation in the same vessel. The concept of a pressure swing reactor that can do reaction and separation in the same vessel has been around for several decades. Purportedly, the first paper to discuss the concept of a PSAR was published in 1987 [1]. Equivalently, the sorption enhanced reaction process (SERP) concept was exploited by Air Products in the 1990’s for H2 production and other processes [2]. However, the SERP concept first appeared in the literature in 1868, as reported by Rodrigues, et al. [3]! SER or PSAR processes have yet to be commercialized, although several patents have been issued and some novel approaches have been tested at various scales.
A pressure swing adsorption reactor (PSAR) combines a pressure swing adsorption (PSA) process and a heterogeneous catalytic reaction in such a way that a higher conversion can be achieved via Le Chatelier's principle. The difficulty is to find a suitable adsorbent that operates at or near the conditions of the catalytic reactor, i.e., at high temperature and pressure. High pressure is conducive to adsorption, but high temperature is not. In fact, most adsorbents are regenerated at temperatures far below the conditions of a catalytic reactor. Nevertheless, there are some catalytic systems that present an excellent opportunity for the design of a PSAR. The synthesis of NH3 from H2 and N2 is one such system.
Initial process simulations of a PSAR utilizing a relatively simple but novel PSAR cycle for NH3 production have shown that very high conversions can be obtained by using a commercial catalyst and a commercial adsorbent that reversibly adsorbs ammonia at high temperature. Some preliminary results from a novel PSAR cycle are provided in Table 1 [4]. The conversions range from 88 to 96% with the corresponding NH3 purities ranging from 79 to 88 vol%. These numbers are significantly higher that those typically produced in a conventional NH3 synthesis reactor, with conversions and NH3 purities limited to between 20 and 40%.
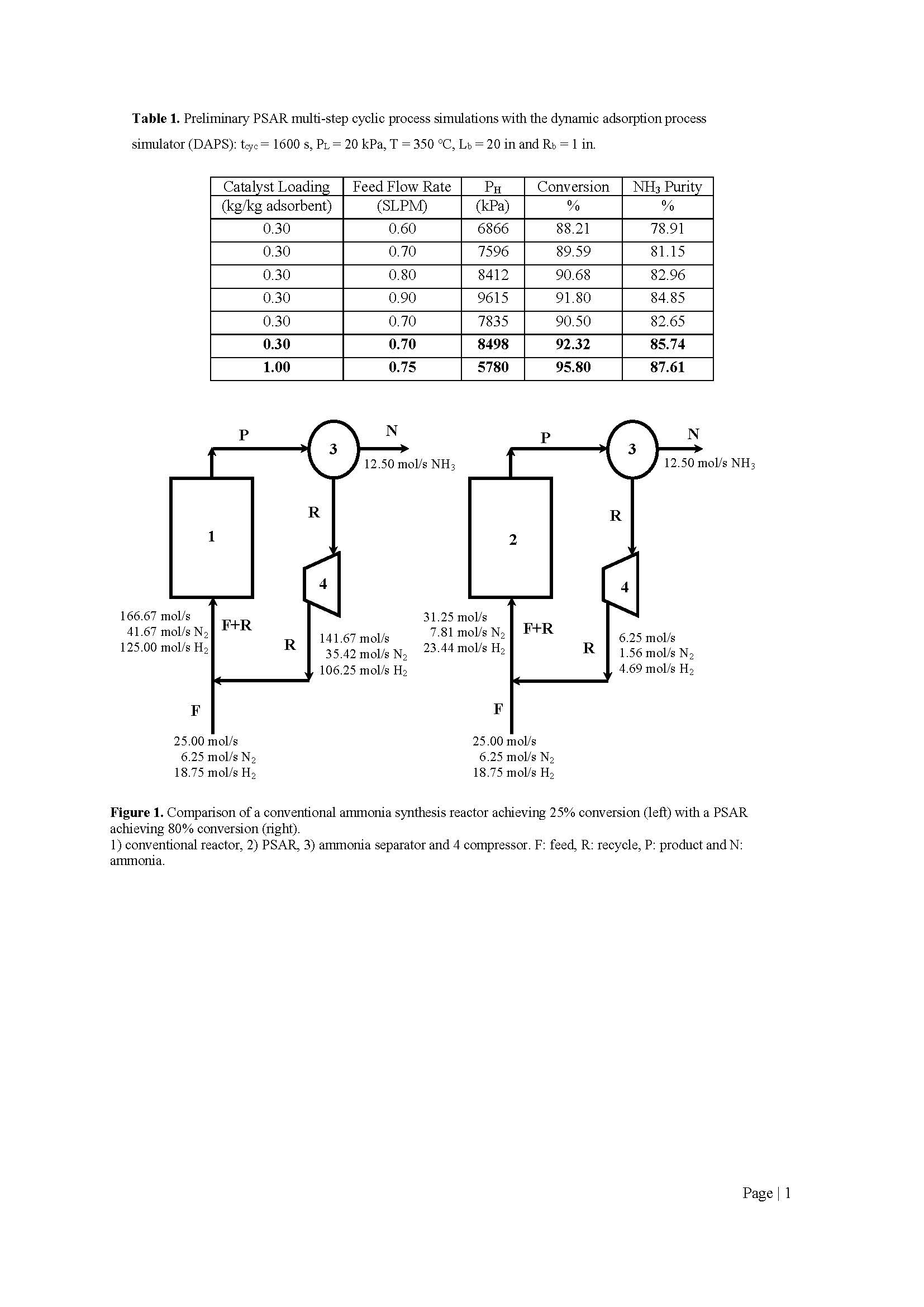
Based on these simulation results, the impetus behind a PSAR is shown in Figure 1, which compares a conventional ammonia synthesis reactor with an assumed 20% conversion to a PSAR with an assumed 80% conversion. For the same raw feed and NH3 production rates, the conventional reactor must recycle 23 times more of the unreacted gas back to the reactor compared to the PSAR. With conversions reaching as high as 96% in the PSAR, as shown in Table 1, the recycle stream might not be necessary.
The goal of this work is to experimentally validate the simulation results in Table 1. For this purpose, a bench-scale PSAR has been set-up that is being used to study 1) rection kinetics of the conversion of H2 and N2 to NH3 under dynamic and steady state conditions using a commercial catalyst, 2) to study the conversion of H2 and N2 to NH3 in the absence and presence of the adsorbent, 3) to carry out PSAR cycles under various conditions to determine their effects on the conversion of H2 and N2 to NH3 in a mixed bed of catalyst and adsorbent.
This presentation will discuss the commercial adsorbent screening study for high temperature NH3 adsorption. It will show the quasi-equilibrium adsorption isotherms on the best NH3 adsorbent. It will introduce a 1-bed PSAR system developed for steady-state and dynamic kinetic studies with commercial and developmental catalysts. It will describe the dynamic and steady-state kinetic experiments with a commercial catalyst. It will discuss the Temkin-Pyzhev (T-P) steady-state and dynamic kinetic models. It will show how well the steady-state and dynamic experiments correlate with the two T-P kinetic models. It will present exciting PSAR cycle simulation results that produce a high concentration of NH3 at high conversion at a modest temperature and especially pressure. Finally, it will provide, for the first time, experimental results that show proof-of-concept for a PSAR using a commercial catalyst and a commercial adsorbent for the production of NH3.
References
[1] G. G. Vaporciyan and R. H. Kadlec, AIChE J., 33, 1334-1343 (1987).
[2] B. T. Carvill, J. R. Hufton, M. Anand and S. Sircar, AIChE J., 42, 2765-2772 (1996).
[3] A. E. Rodrigues, L. M. Maderia, Y.-J. Wu and R. Faria, Sorption Enhanced Reaction Processes, World Scientific, Europe (2018).
[4] J. A. Ritter, A. D. Ebner and C. E. Holland, US Provisional Patent Application (2024).