2025 AIChE Annual Meeting
(146a) Development of Novel Peptide-Based Vaccine Adjuvants Derived from Danger-Associated Molecular Patterns
Authors
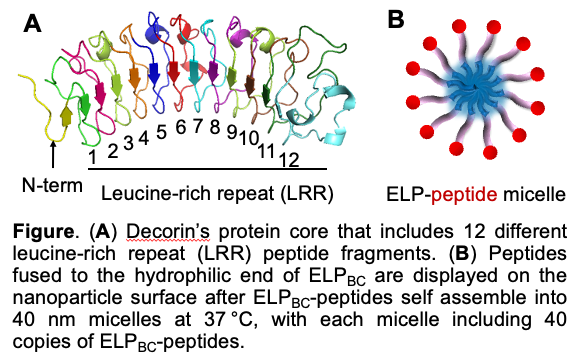
To identify this peptide adjuvant, we harnessed the elastin-like polypeptide (ELP) micelle nanoparticle platform to individually display decorin-derived peptide fragments and compare their immunostimulatory activity. ELPs are bio-inspired, thermos-responsive biopolymers composed of repeating (VPGXG)n sequences, where X is any amino acid except proline. Our rationally designed ELP diblock copolymer (ELPBC) can self-assemble into 40 nm micelle nanoparticles at 37 °C, with each micelle including 40 copies of ELPs. Displaying peptides on this ELP micelle surface does not change the micellar size, shape, and aggregation number. We genetically fused each of decorin’s twelve leucine-rich repeats (LRRs), along with its terminal peptide fragments, to the hydrophilic end of ELPBC. These ELPBC-peptide fusions were recombinantly synthesized at a low cost and spontaneously formed uniform micelle nanoparticles at 37 °C with 40 copies of peptide displayed on each micelle.
We first screened this library of ELPBC micelles displaying decorin-derived peptides using bone marrow-derived dendritic cells (BMDCs), and we identified that LRR8 induced strong Th1-type immune activation, comparable to full-length decorin protein. This activation was confirmed to be TLR2/4-dependent, as treatment with specific inhibitors of TLR2 and TLR4 abolished both LRR8- and decorin-induced immune activation in BMDCs. Molecular docking further revealed that LRR8 binds to a distinct domain on TLR2/4, separate from the lipopolysaccharide (LPS)-binding region, suggesting a different receptor engagement mechanism with potentially improved safety compared to LPS, a bacterial TLR2/4 agonist known for its toxicity. Subsequently, we assessed the in vivo efficacy of this novel peptide-based adjuvant. C57BL/6 mice were immunized subcutaneously twice with mixed micelles containing ELPBC-LRR8 (adjuvant) and ELPBC-OVA (ovalbumin, antigen). This formulation significantly enhanced anti-OVA antibody production compared to mice immunized with ELP-OVA micelles alone or in combination with monophosphoryl lipid A (MPLA), a conventional TLR4 agonist adjuvant derived from PAMPs.
In summary, we report the successful identification and validation of LRR8, a decorin-derived DAMP peptide that acts as a TLR2/4 agonist and demonstrates its potent adjuvant activity in vitro and in vivo. This study highlights the potential of developing a novel class of peptide-based vaccine adjuvants from endogenous danger signals and it also demonstrates the efficiency of using ELP micelle nanoparticles as both screening and delivery platforms for development of DAMP-derived immunostimulatory peptides.