2025 AIChE Annual Meeting
(41h) Development of an Integrated Continuous Manufacturing (ICM) Process for an Antiviral Drug
Author
Stephen Born - Presenter, CONTINUUS Pharmaceutical
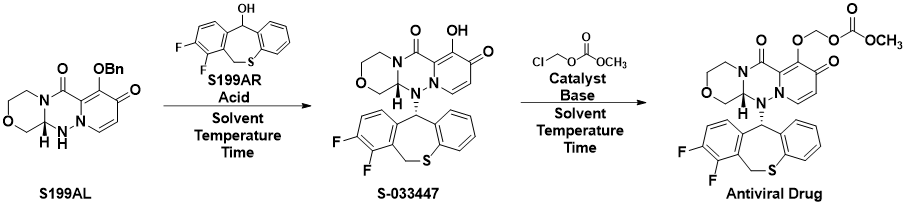
The development of a continuous process to produce an antiviral drug for the treatment of influenza is presented. The motivation behind this effort is to transition towards the integrated continuous manufacturing (ICM) as a platform to create an agile and on-demand supply chain capability for a drug that has a high variable market demand. The work described herein includes an improved late-stage synthetic route to the API that: (1) reduced a four-step synthesis down to two-steps, (2) identified key diastereomeric crystallization conditions to reach required material specifications; and (3) eliminated the use of N-methyl-2-pyrrolidone and N,N-dimethylacetamide in exchange for a more environmentally benign solvent mixture of acetonitrile and water. After a full revision of the two-step synthetic route in batch mode, each unit operation was translated to continuous mode to evaluate their performance. together, this key synthetic route eliminated 16 unit operations, reduced lead time from 12 months to two days, and significantly simplified the process.