2025 AIChE Annual Meeting
(64b) Designing Electrolytes for Next-Generation Batteries and Electrolyzers
Author
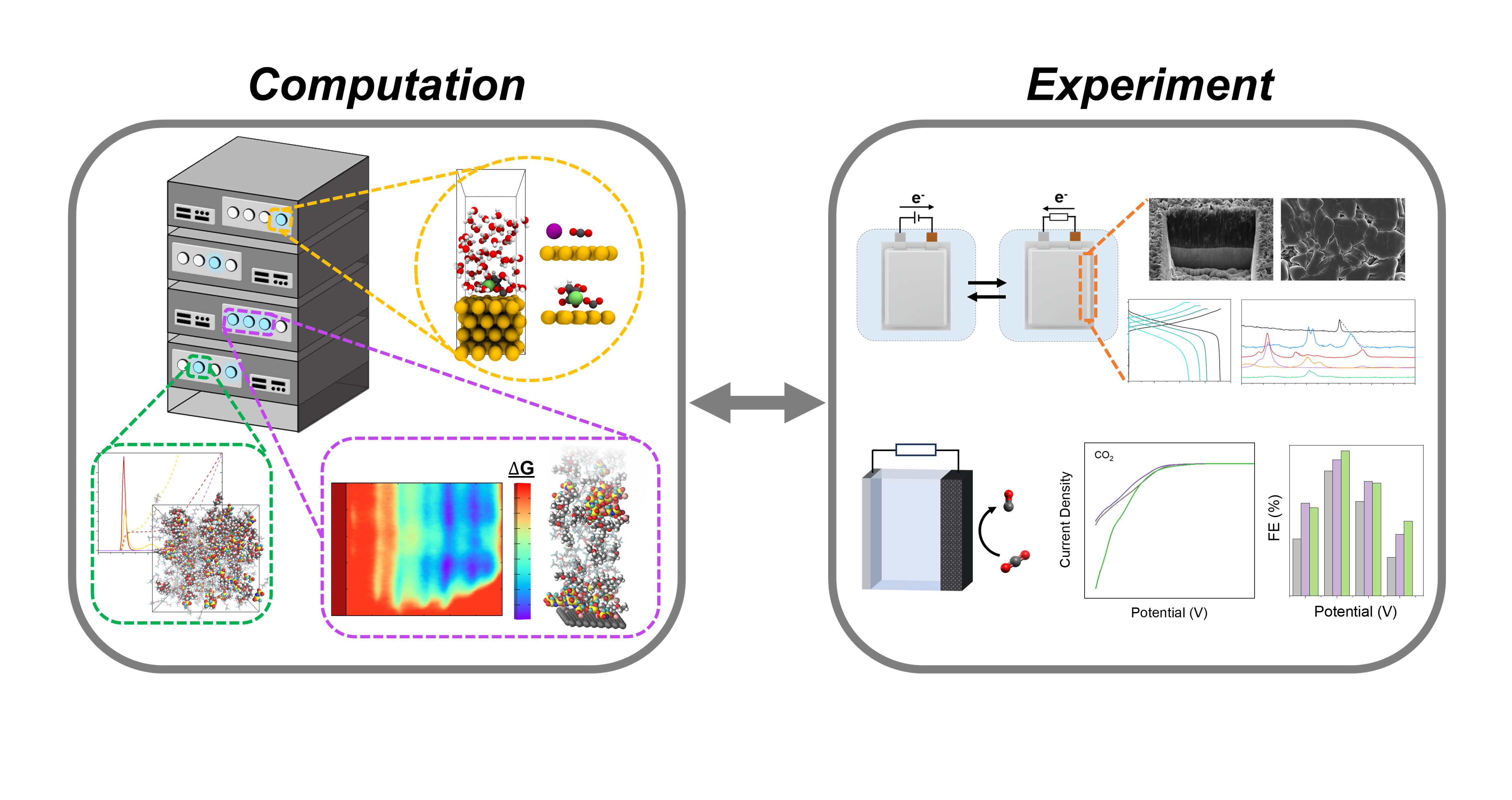
Previously, we investigated the effect on Li+ coordination environment on the performance of Li metal batteries under kinetic stress, such as reduced temperatures and high-power charging. Specifically, we found that inducing ion-pairing between Li+ and anions reduces the desolvation energy faced by Li+ in the electric double-layer. This was probed using experimental and computational means, including the application of free-energy sampling methods. The insights gained from these studies were leveraged to develop electrolytes for both sulfur-type and > 4 V Li metal full batteries capable of cycling down to -60 oC and at elevated charging rates.
More recently, we also applied this hybrid computational/experimental methodology to electrolyte design for Zinc batteries. We demonstrate that pairing between Zn2+ and halide anions can significantly improve the poor Zn deposition kinetics typically found in organic solvents, while also undercutting the costs of state-of-the-art systems. Deposition from these systems generates polyhedral, textured Zn and exceptional Coulombic efficiencies without any discernable corrosion.
Lastly, we demonstrate that electrolyte composition and structure can also be tuned to enhance CO2 electrolysis. These electrolytes are based on multivalent cations that are stabilized in the bicarbonate buffer solutions common in CO2 electrolyzers with electrolyte additives. Electrolytes based on these chemistries show increased electrochemical activity for CO2 reduction relative to hydrogen evolution. Ab-initio molecular dynamics (AIMD) simulations reveal that these additives polarize H2O away from the cathode and that multivalent cations induce greater activation of *CO2 than standard monovalent cations.
This talk showcases a combined experimental and computational approach to electrolyte design for the advancement of electrochemical energy storage and conversion.