2025 AIChE Annual Meeting
(584t) Design Criteria for Direct Seawater Electrolysis Integrated with in-Situ Water Purification
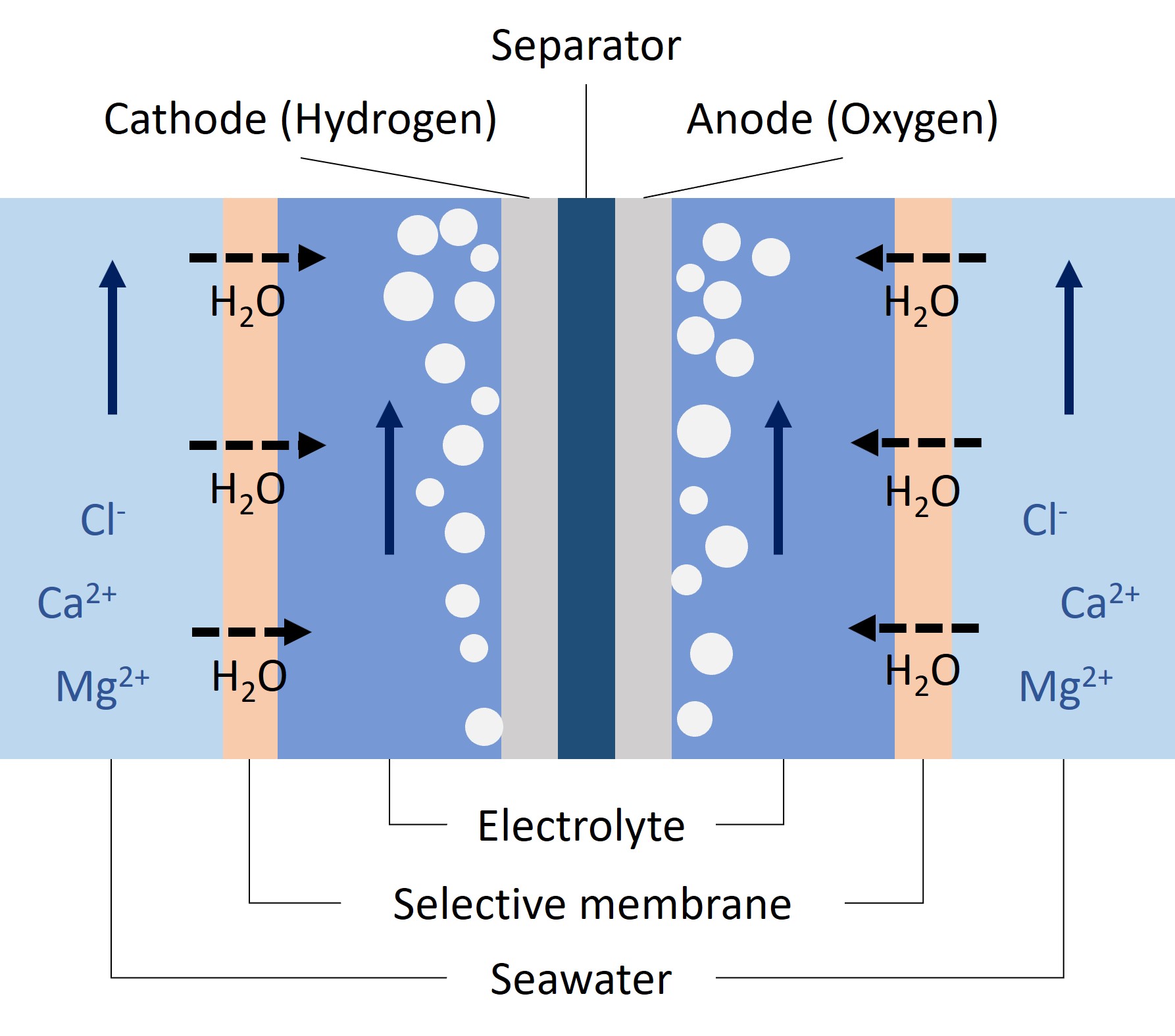
Conventional electrolysis systems use energy-intensive pretreatment steps, including reverse osmosis and ion exchange, to remove these impurities. To eliminate these processes, this project explores integrating in-situ water purification into electrolyzer using selective membranes—specifically forward osmosis (FO) and PTFE membranes. These membranes leverage osmotic or vapor pressure gradients to transport water into the electrolyzer while excluding unwanted ions, thus simplifying system design and energy demands.
We developed a zero-gap water electrolyzer flow cell incorporating these membranes and quantified water transport rates via weight-change measurements to determine current density limits for steady-state operation. The selectivity of these membranes was evaluated using ion chromatography. Long-term performance and degradation were assessed over 40 hours through electrochemical and materials analysis.
Results show that electrolyzers with FO membranes experienced pH imbalances between anolyte and catholyte due to sodium ion dominance, which suppressed proton migration. This caused acidity buildup, leading to anode corrosion and catalyst redeposition on the cathode. These effects highlight a critical limitation of the systems using FO membranes in near-neutral pH conditions.
In contrast, systems using PTFE membranes demonstrated stable performance over 40 hours, with no significant degradation observed. Our findings identify key design parameters and limitations for scaling up in-situ purification strategies in seawater electrolysis, offering a pathway toward stable offshore hydrogen production without the need for traditional water pretreatment.