2025 AIChE Annual Meeting
(652f) Design of Conditionally Active Cytokine Mimetics Targeting Amyloid-Beta Plaques to Modulate the Immune Landscape in Alzheimer’s Disease
Authors
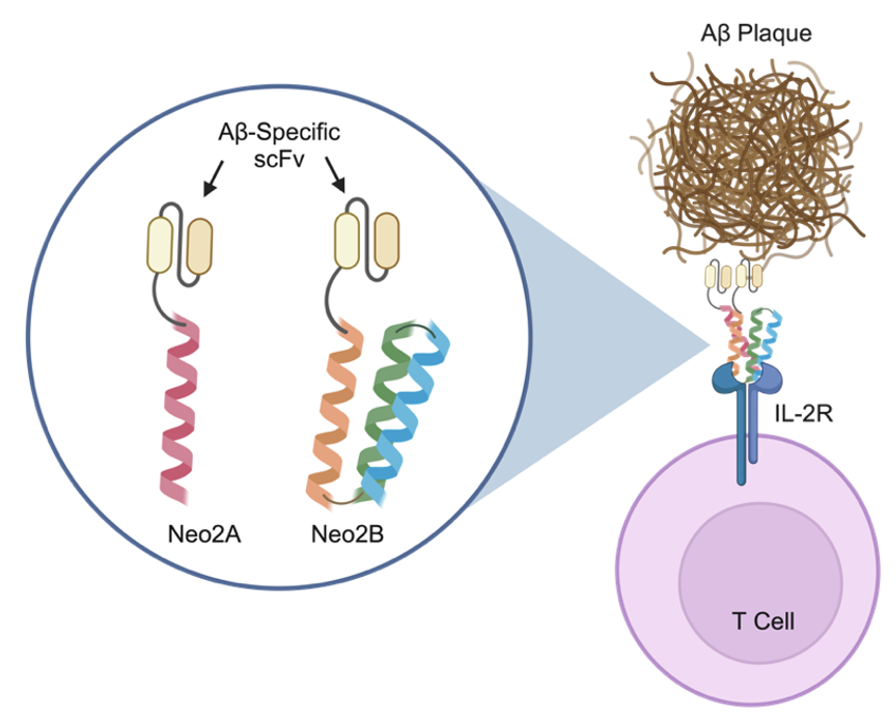
There is growing interest in capitalizing on immune cell populations naturally present in the brain to regulate inflammatory processes that are linked to AD pathogenesis. Our team helped to develop a de novo computationally designed mimetic of the interleukin-2 (IL-2) cytokine, denoted Neo-2. Like natural IL-2, Neo-2 potently induces T cell activation and proliferation, making it an attractive candidate for stimulation of T cells in the brain.5,6 Due to the hyperstability of Neo-2, we demonstrated the capacity to design a split, conditionally active version of Neo-2, such that colocalization of two split fragments is required to successfully signal.6,7 Building on this promising result, the goal of this research is to engineer cytokine-antibody fusion proteins (immunocytokines) that effect specific recruitment and activation of T cells at the site of Aß plaques in the brain. To achieve this, we formulated immunocytokines consisting of an antibody single-chain variable fragment (scFv) against oligomerized Aß fused to each of the two split fragments of Neo-2 (Figure 1).
Four split fusion proteins were designed and expressed in Expi293 human embryonic kidney (HEK) cells via transient transfection. Each split fusion protein comprises an scFv that binds the oligomeric (but not the monomeric) form of Aß fused to the N-terminus of a Neo-2 split fragment (either Neo2A or Neo2B).7 Biolayer interferometry-based binding studies demonstrated that our engineered split fusion proteins engage the IL-2 receptor (IL-2R) when administered simultaneously but not independently. Flow cytometry studies demonstrated that the split fusion proteins activate IL-2 signaling on YT-1 human natural killer cells when combined but not alone, reinforcing their conditional activity. We also showed that our engineered split fusion proteins bound to immobilized Aß aggregates in enzyme-linked immunosorbent assays (ELISAs), verifying the functionality of the scFvs in the context of the fusion proteins. In addition, YT-1 signaling studies on Aß aggregate-coated plates revealed that split fusion proteins colocalize on Aß oligomers and that simultaneous binding to Ab and activation of immune cell signaling can be achieved. Lastly, when exposed to Aß oligomers in brain sections isolated from 5xFAD mice, both Neo2A- and Neo2B-containing split fusion proteins home to areas where plaques are present.
Having established proof of concept for conditional activation of immune cells in the vicinity of Aß plaques, we are now focusing on the following questions: (1) Can we successfully deliver our immunocytokines to the brain? (2) Can both Neo-2 fragments colocalize to Aß plaques to reconstitute IL-2 signaling at the site of disease in vivo? and (3) Does localization to Aß plaques and activation of IL-2 signaling lead to recruitment and stimulation of T cells in the brain? Demonstrating the immunomodulatory functions of our engineered split fusion proteins in the brain will inspire the design of novel immunocytokines targeting other immune cell populations, such as microglia, astrocytes, and perivascular macrophages. Overall, our innovative approach provides a blueprint for designing targeted, conditionally active immune interventions that can be used in AD and a host of other neurological conditions.
Figure Caption: Engineered amyloid-beta-targeted conditionally active cytokine mimetics to modulate the immune environment at the site of lesions in Alzheimer’s Disease. Split fragments of the de novo computationally designed cytokine mimetic Neo-2 (Neo2A and Neo2B) colocalize to the IL-2 receptor (IL-2R) complex on the surface of T cells. These two artificial IL-2 fragments are C-terminally fused to amyloid-beta (Aß) oligomer-specific antibody single-chain variable fragment (scFv) regions. The goal of these engineered split fusion proteins is to achieve recruitment and activation of specific immune cell populations the site of Aß plaques for targeted treatment of Alzheimer’s Disease. Created using Biorender (biorender.com).
Works Cited
- Hou Y, Dan X, Babbar M, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15(10):565-581. doi:10.1038/s41582-019-0244-7
- Reif A, Schmitt A, Fritzen S, Lesch KP. Neurogenesis and schizophrenia: dividing neurons in a divided mind? Eur Arch Psychiatry Clin Neurosci. 2007;257(5):290-299. doi:10.1007/s00406-007-0733-3
- Drolle E, Hane F, Lee B, Leonenko Z. Atomic force microscopy to study molecular mechanisms of amyloid fibril formation and toxicity in Alzheimer’s disease. Drug Metab Rev. 2014;46(2):207-223. doi:10.3109/03602532.2014.882354
- Panza F, Lozupone M, Logroscino G, Imbimbo BP. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat Rev Neurol. 2019;15(2):73-88. doi:10.1038/s41582-018-0116-6
- Silva DA, Yu S, Ulge UY, et al. De novo design of potent and selective mimics of IL-2 and IL-15. Nature. 2019;565(7738):186-191. doi:10.1038/s41586-018-0830-7
- Yang H, Ulge UY, Quijano-Rubio A, et al. Design of cell-type-specific hyperstable IL-4 mimetics via modular de novo scaffolds. Nat Chem Biol. 2023;19(9):1127-1137. doi:10.1038/s41589-023-01313-6
- Quijano-Rubio A, Bhuiyan AM, Yang H, et al. A split, conditionally active mimetic of IL-2 reduces the toxicity of systemic cytokine therapy. Nat Biotechnol. 2023;41(4):532-540. doi:10.1038/s41587-022-01510-z