2025 AIChE Annual Meeting
(461d) A Density Functional Theory Study on Bimetallic Transition-Metal-Doped CeO? Catalysts for the Reverse Water Gas Shift Reaction
Authors
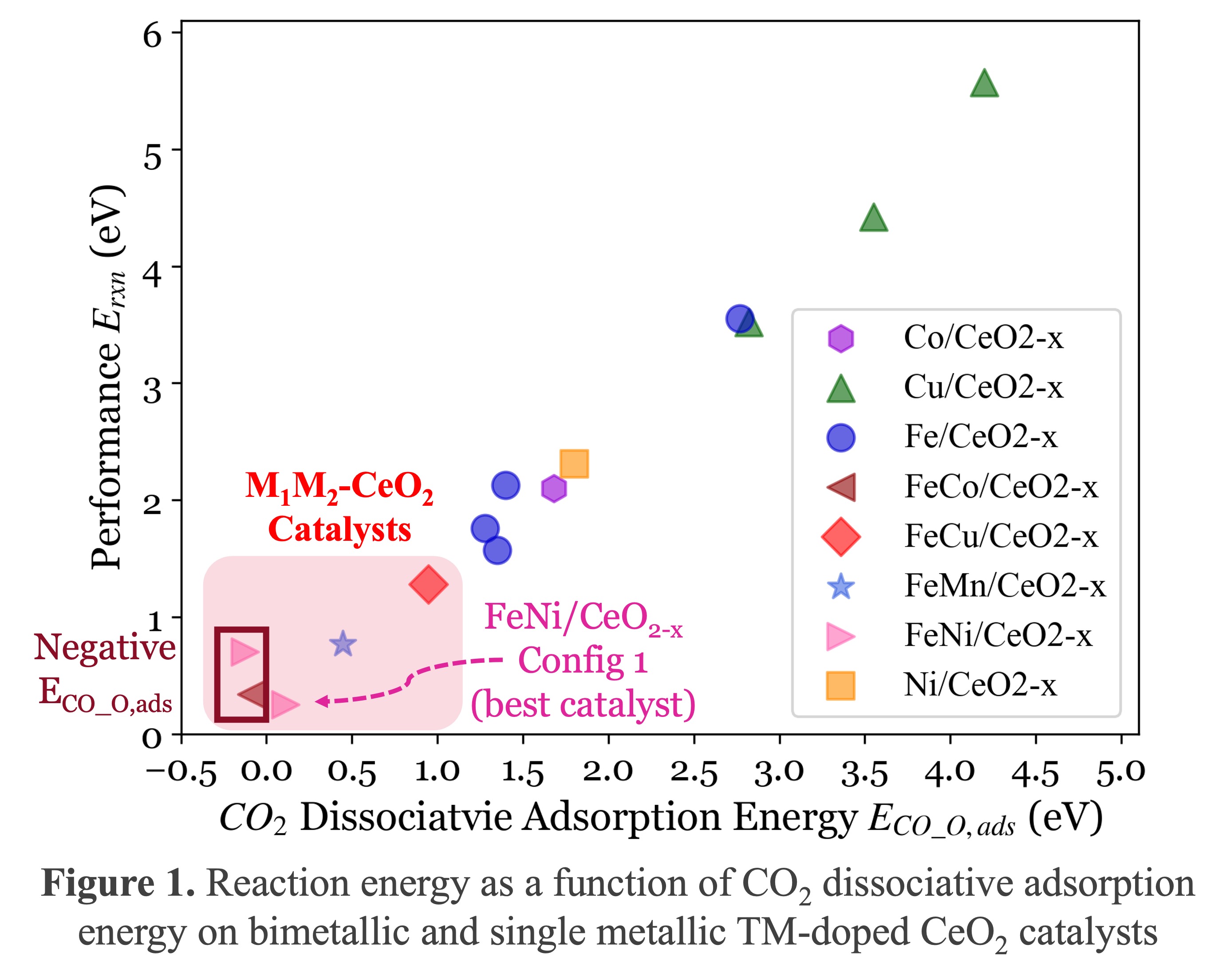
To bridge this gap, this study conducted a theoretical investigation using density functional theory (DFT) calculations on bimetallic TM-doped-CeO2 (M1M2-CeO2) catalysts (M1=Fe; M2=Co, Ni, Mn, and Cu) to further optimize CeO2’s surface structure for the RWGS reaction. Oxygen vacancy (OV) was considered to improve CO2 reduction efficiency. To the authors’ knowledge, this theoretical study is the first to consider bimetallic TM-doped-CeO2 for the RWGS reaction. The investigation focused on the direct reduction of CO2 to CO on catalyst surfaces by employing a four-step DFT calculation process: (1) bimetallic-doping to pure CeO2 (111) surface, (2) OV creation, (3) CO2 adsorption, and (4) CO2 dissociation.
Given the time-consuming nature of transition state calculations and observed linear Brønsted-Evans-Polanyi relationships, reaction energy was used as a catalyst performance descriptor. Results (Figure 1) indicate that bimetallic M1M2-CeO2 catalysts outperformed single-metallic TM-doped-CeO2 catalysts reported in the literature, exhibiting significantly lower reaction and CO2 dissociative adsorption energies, thus reducing energy barriers and enhancing efficiency and feasibility for the RWGS reaction. Moreover, two bimetallic catalysts achieved negative CO2 dissociative adsorption energies, making Step (4) spontaneous and potentially enhancing cost-efficiency for industrial-scale applications by eliminating heating requirements for that step. Overall, these results highlight the promising potential of bimetallic TM-doped-CeO2 catalysts in enhancing catalytic performance for the RWGS reaction.