2025 AIChE Annual Meeting
(25a) Degradable Cyclic Amino Alcohol Ionizable Lipids As Vectors for Potent Influenza mRNA Vaccines
Authors
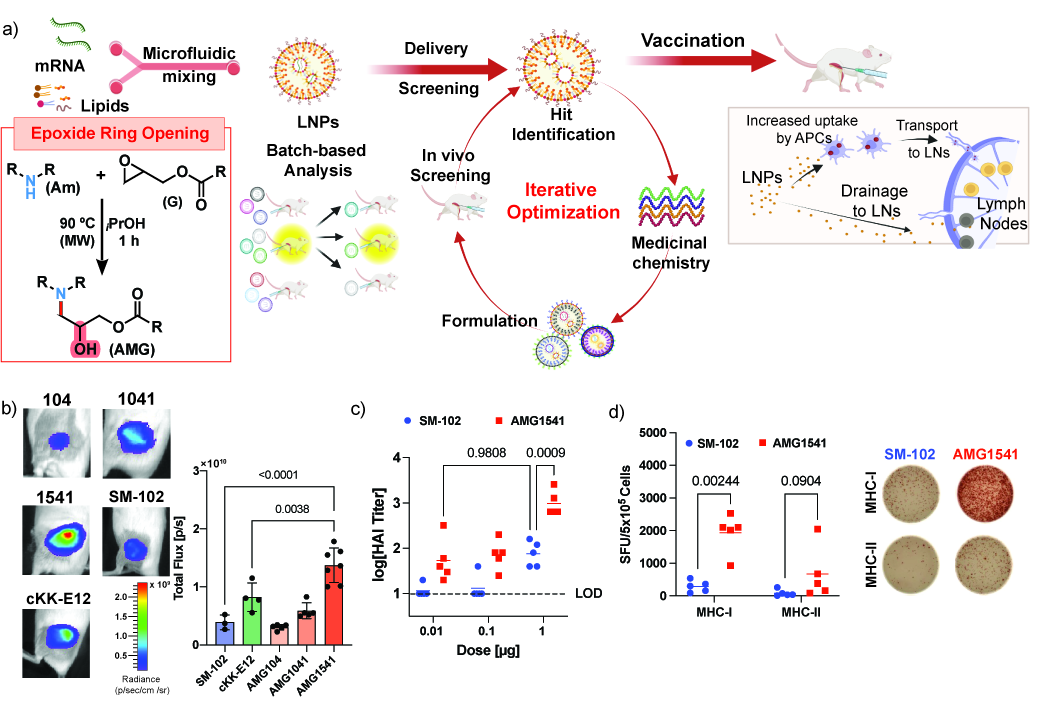
RNA vaccines have revolutionized global health, with over 2 billion doses administered. Enhancing their potency could improve immunity at lower doses, reducing manufacturing challenges and side effects. Ionizable lipids are key to boosting vaccine potency by facilitating endosomal escape, enhancing immunogenicity, and improving tolerability. While current lipids like SM-102 set benchmarks, advancements beyond FDA-approved formulations remain limited. To address this, we aim to develop more potent ionizable lipids to enhance protein production within APCs, improving vaccine safety and efficacy. In this work, we utilize a hybrid rational design and combinatorial approach to develop a structurally unique series of ionizable lipids for mRNA vaccination. We developed an initial combinatorial ionizable lipid library using nucleophilic attack of aliphatic epoxide tails by amines, as this has previously been utilized to generate the highly potent ionizable lipids. Here, we build upon these previous efforts by adding biodegradability via a high-yield reaction between “clickable” glycidyl ester functionalized hydrocarbon tails and constrained, cyclic secondary amines. Subsequently, we optimized the molecular structure of top ionizable lipid using medicinal chemistry approach and perform structure-immunogenicity studies to generate potent vaccine LNP formulations (Figure 1a).
Results
Systematic optimization identified AMG1541, a next-generation lipid that achieves 4.5-fold higher mRNA expression than AMG104 and significantly outperforms benchmark lipids SM-102 and cKK-E12 (Figure 1b). AMG1541 combines superior potency with favorable pharmacokinetics, featuring biodegradable glycidyl ester tails that promote rapid clearance from the injection site and reduced liver accumulation, minimizing hepatotoxicity risks. Notably, AMG1541 LNPs show 8.3-fold lower mRNA expression in the liver while delivering 3.5-fold higher expression at the injection site compared to SM-102. In mice, AMG1541 achieves comparable neutralizing immunity to SM-102 at 100-fold lower doses, offering potential cost and side-effect reductions(Figure 1c). Moreover, AMG1541 induced 6.7-fold higher CD8 T-cell response as compared SM-102 lipids (Figure 1d). Enhanced lymph node drainage and improved antigen expression in APCs amplify germinal centers and antibody titers, underscoring AMG1541’s ability to improve mRNA delivery, vaccine tolerability, and adaptive immune responses for next-generation vaccines.
Conclusion
The mRNA vaccine formulations developed in this work significantly enhance vaccine potency, providing valuable structural insights that could inform the design of next-generation delivery systems. These findings on the relationship between LNP chemistry and RNA delivery hold promise for guiding the development of advanced ionizable lipids for improved delivery efficiency.