2025 AIChE Annual Meeting
(86g) Deep Tissue In Vivo Cell Tracking of Micrometastatic Peritoneal Tumors
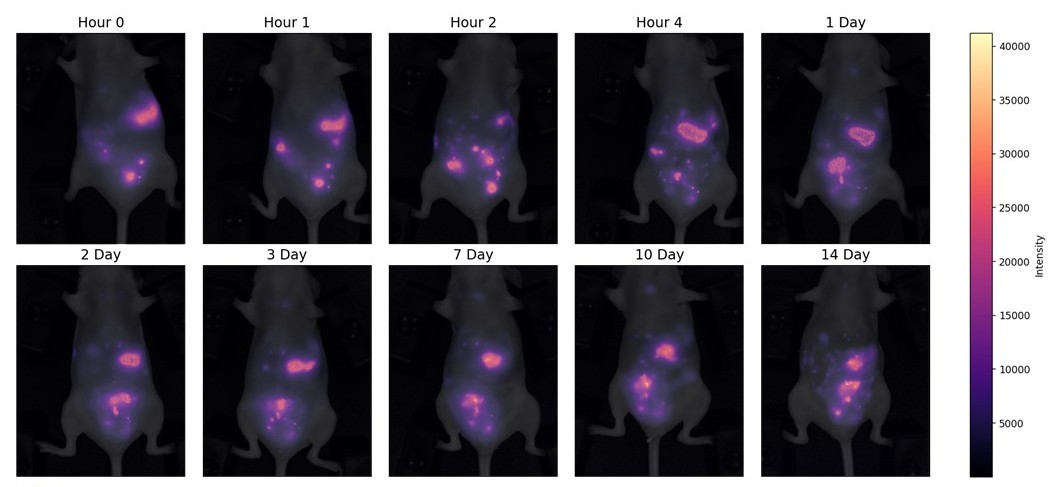
Cell tracking is a powerful method that provides insights into cell fate, proliferation, and migration. Currently, fluorescent in vivo cell tracking is greatly limited by tissue penetration depth. Shortwave infrared (SWIR) imaging (1000–1700 nm) enhances penetration and resolution by minimizing tissue absorbance, autofluorescence, and light scattering. In this wavelength range, quantum dots (QDs) have emerged as superior labeling and imaging agents due to their emission tunability, photostability, and brightness. PbS/CdS (core/shell) dihydrolipoic acid (DHLA)-capped QDs were used to ex vivo label GFP-expressing OVCAR5 cells, a disseminated peritoneal cancer line. Non-specific uptake of DHLA-capped QDs was optimized for maximum uptake without sacrificing cellular viability. Following intraperitoneal injection, QD-labeled GFP-OVCAR5 cells were tracked in vivo for 14 days, enabling identification of individual micrometastases and migration patterns. Metastases appear to travel away from the peritoneal cavity into the mediastinal region within 4 hours of injection. After 14 days of in vivo tracking, ex vivo imaging confirmed persistent individual cell labeling through GFP and QD signal overlap. This study demonstrates the feasibility of QD-based direct labeling for long-term, high-resolution cell tracking in real time.