2025 AIChE Annual Meeting
(111f) Deciphering Unresolved Capacity Loss at Lithium Metal Anodes through Quantitative Analysis of Li Residual Materials
Authors
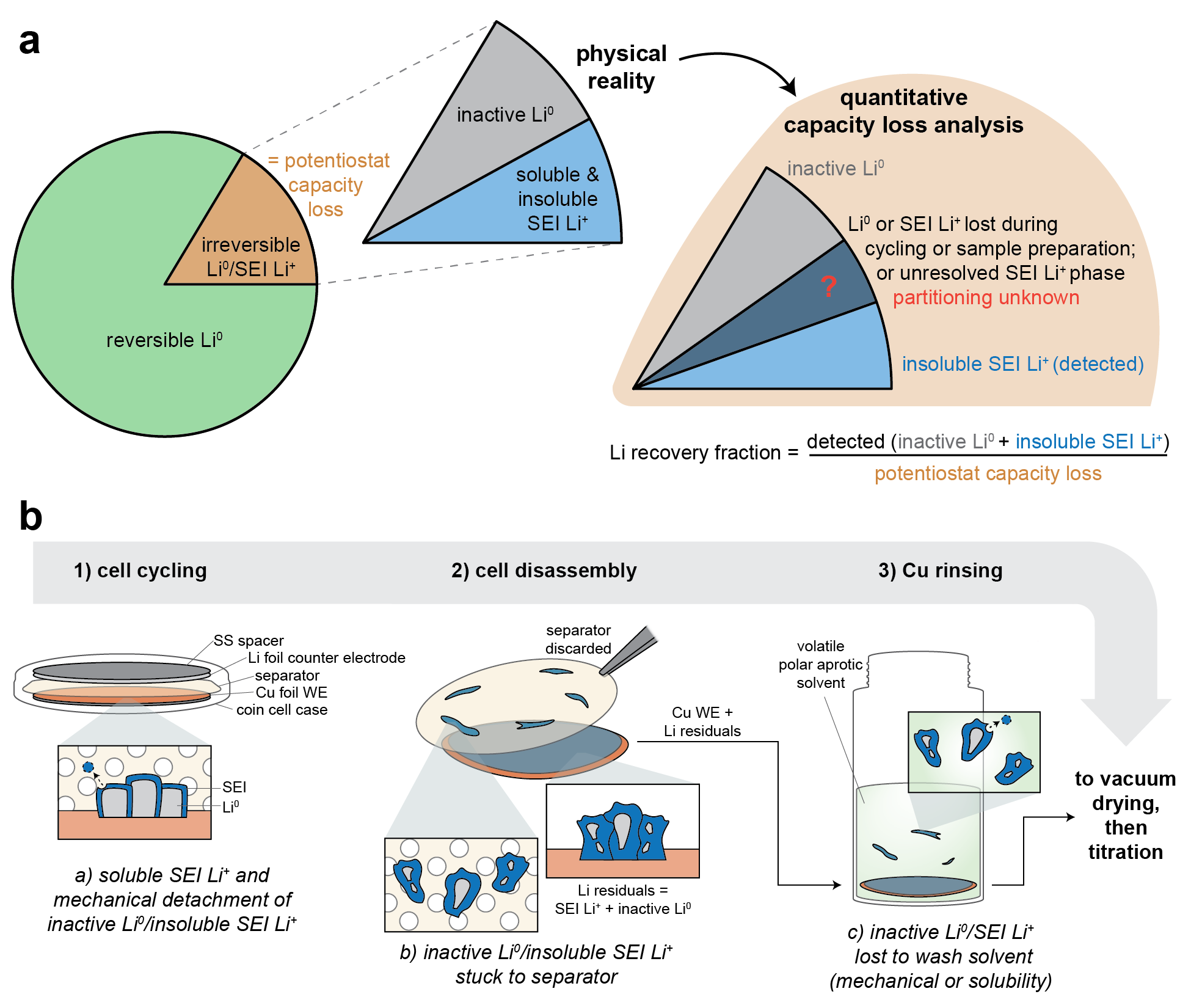
While these techniques are a powerful approach to understanding the mechanisms by which interventions like additives6 and pressure7 improve capacity retention, and to correlate the prevalence of certain SEI species with CE5, integrating multiple titrations to ever more completely map capacity loss presents certain challenges. For one, any deficit between measured Li inventory and total capacity loss could originate from material losses during cycling or sample preparation, or they could simply represent materials not yet chemically-resolvable by titrations (Figure 1a). Additionally, quantified species are usually normalized, but there can be ambiguity in normalization that impacts the proportional breakdown of certain capacity loss modes (Figure 1a). Here, we systematically test the significance of these issues by first developing techniques to directly measure the material losses at different stages of sample preparation (Figure 1b), then investigate impacts of the normalization procedure. We also critically assess the reliability of our findings through the replication of experiments by different co-authors at separate institutions.
This work enables closer scrutiny of the significance of unresolvable material losses during the quantitative analysis of Li anode residuals, testing the boundaries of these techniques. We ultimately found that attempting to track all irreversible Li0/Li+ inventory does not impact conclusions related to trends in SEI composition and capacity loss modes between four disparate electrolytes. This work demonstrates the robustness of capacity loss analysis and SEI quantification techniques to minor losses of material during sample processing, and proposes a more stringent normalization procedure that can be adopted when quantitative material accounting is of particular concern.
Figure 1. (a) Schematic of capacity loss partitioning as measured by quantitative capacity loss analysis techniques. (b) Schematic of sample preparation process for SEI quantifications (steps 1-3) and possible modes of material loss (list a-c).
References
1 Hobold, G. M. et al. Moving beyond 99.9% Coulombic efficiency for lithium anodes in liquid electrolytes. Nat. Energy 6, 951-960 (2021). https://doi.org/10.1038/s41560-021-00910-w
2 Fang, C. et al. Quantifying inactive lithium in lithium metal batteries. Nature 572, 511-515 (2019). https://doi.org/10.1038/s41586-019-1481-z
3 McShane, E. J. et al. Quantification of Inactive Lithium and Solid-Electrolyte Interphase Species on Graphite Electrodes after Fast Charging. ACS Energy Letters 5, 2045-2051 (2020). https://doi.org/10.1021/acsenergylett.0c00859
4 Hobold, G. M. & Gallant, B. M. Quantifying Capacity Loss Mechanisms of Li Metal Anodes beyond Inactive Li-0. ACS Energy Lett. 7, 3458-3466 (2022). https://doi.org/10.1021/acsenergylett.2c01845
5 Hobold, G. M., Wang, C., Steinberg, K., Li, Y. & Gallant, B. M. High lithium oxide prevalence in the lithium solid–electrolyte interphase for high Coulombic efficiency. Nat. Energy (2024). https://doi.org/10.1038/s41560-024-01494-x
6 Steinberg, K. & Gallant, B. M. Revealing the Role of Lithium Carbonate at Lithium Metal Anodes Through Study of Gas-Reacted Interphases. J. Electrochem. Soc. 171, 080530 (2024).
7 Fang, C. et al. Pressure-tailored lithium deposition and dissolution in lithium metal batteries. Nat. Energy 6, 987-994 (2021). https://doi.org/10.1038/s41560-021-00917-3