2025 AIChE Annual Meeting
(7c) On Deactivation Mechanisms of Multifunctional Beta Zeolite Catalysts during Ethanol Upgrading to C3+ Olefins
Authors
Young Gul Hur, Purdue University
Stephen Purdy, Oak Ridge National Laboratory
Meijun Li, Oak Ridge National Laboratory
Andrew D. Sutton, Oak Ridge National Laboratory
Brandon Bukowski, Johns Hopkins University
James Harris, University of Alabama
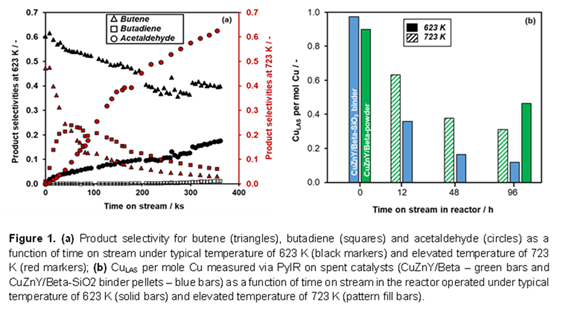
Ethanol can be upgraded to jet fuel by first forming butene-rich product streams, followed by oligomerization and hydrotreating. Circumventing the energy intensive dehydration of ethanol to ethylene by instead forming butene directly from ethanol is projected to lower the lifecycle emissions of ethanol-to-jet processes. Beta zeolite impregnated with metals (i.e., Cu, Zn, and Y; CuZnY/Beta), is a multifunctional catalyst wherein Cu facilitates the dehydrogenation of ethanol to acetaldehyde. Lewis acidic Y sites are responsible for C-C coupling between two acetaldehyde molecules to form crotonaldehyde, which is subsequently hydrogenated to butanal over Cu. Finally, butanal undergoes further hydrogenation to butanol, and dehydration to form butene. Reactivity and selectivity of CuZnY/Beta catalysts are sensitive to the pretreatment and reaction conditions. At elevated reaction temperatures (i.e., 723 K) higher selectivity to acetaldehyde is observed along with a transition from butene to butadiene selectivity with extended time on stream (TOS) (~ 96 h). However, at a reaction temperature of 623 K, there is minimal butadiene formation over time on stream, suggesting that elevated reaction temperatures are detrimental to catalyst stability and to olefin selectivity. We characterized the spent catalysts by multiple techniques (e.g., chemisorption IR, XRD, Raman, TEM, TGA, SSNMR, XAS) to study the source of the deactivation. Carbon deposition was determined to be the primary source of deactivation, and we characterized the nature and extent of coke formation as function of reaction temperature and TOS during ethanol dehydrogenation. Repeating these studies on CuZnY/Beta catalyst formulated into pellets using a SiO2 binder provided similar insights. Co-feeding potential coke precursors demonstrated that cyclic oxygenates lead to side reactions which lead to loss of active sites for butene formation, consistent with their strong binding energies from computational studies. These studies collectively guide ongoing research in developing robust multifunctional catalysts for sustainable aviation fuel production.