2025 AIChE Annual Meeting
(624g) Coupled Radial Basis Function Neural Network, Mechanistic Modeling and Computational Fluid Dynamics for Efficient Lipid Nanoparticle Formulation in Microfluidic Devices
Authors
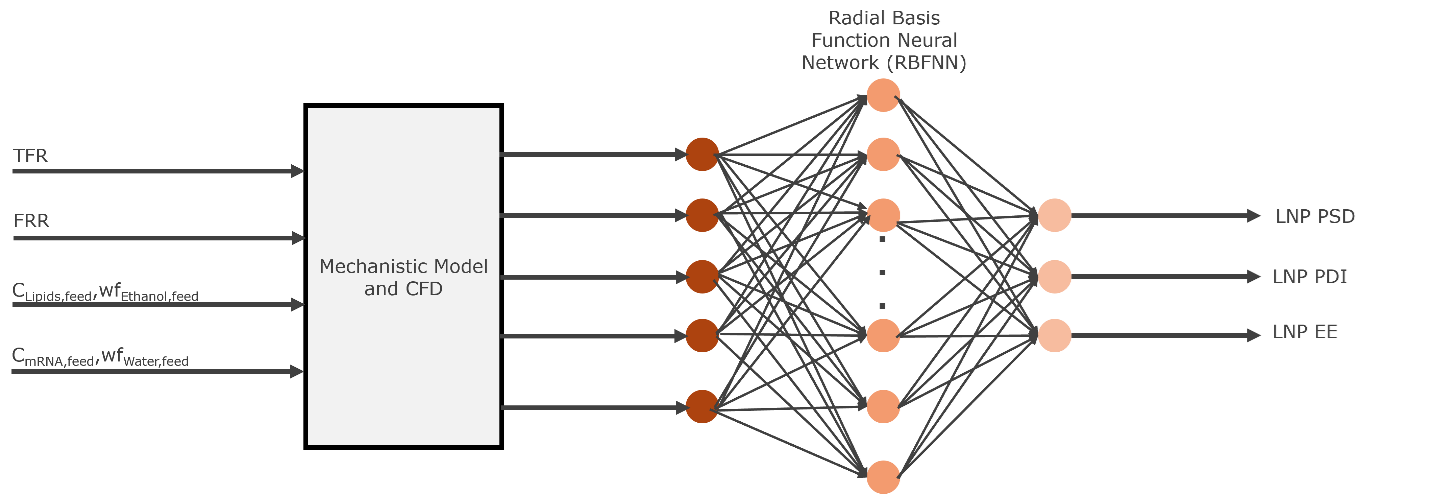
This work introduces a hybrid modeling framework that combines Computational Fluid Dynamics (CFD) with Radial Basis Function Neural Networks (RBFNN) to predict and optimize LNP formation in continuous microfluidic devices. The digital twin captures complex interactions between formulation parameters, fluid flow, and mixing dynamics, linking them to critical quality attributes (CQAs) such as particle size, polydispersity, and encapsulation efficiency.
Mechanistic simulations are used to extract key flow and species features, which inform the training of RBFNNs. These serve as surrogate models for rapid prediction and optimization. Experimental data validate the models, ensuring credibility and alignment with regulatory expectations.
The broader impact lies in accelerating process development and supporting Quality-by-Design (QbD) by integrating mechanistic insight with machine learning. This approach lays the foundation for digital twins that can inform real-time control, model-based scale-up, and advanced biomanufacturing strategies.