2025 AIChE Annual Meeting
(691a) Converting Waste “Single-Use” Plastic into Graphitic Carbons
Authors
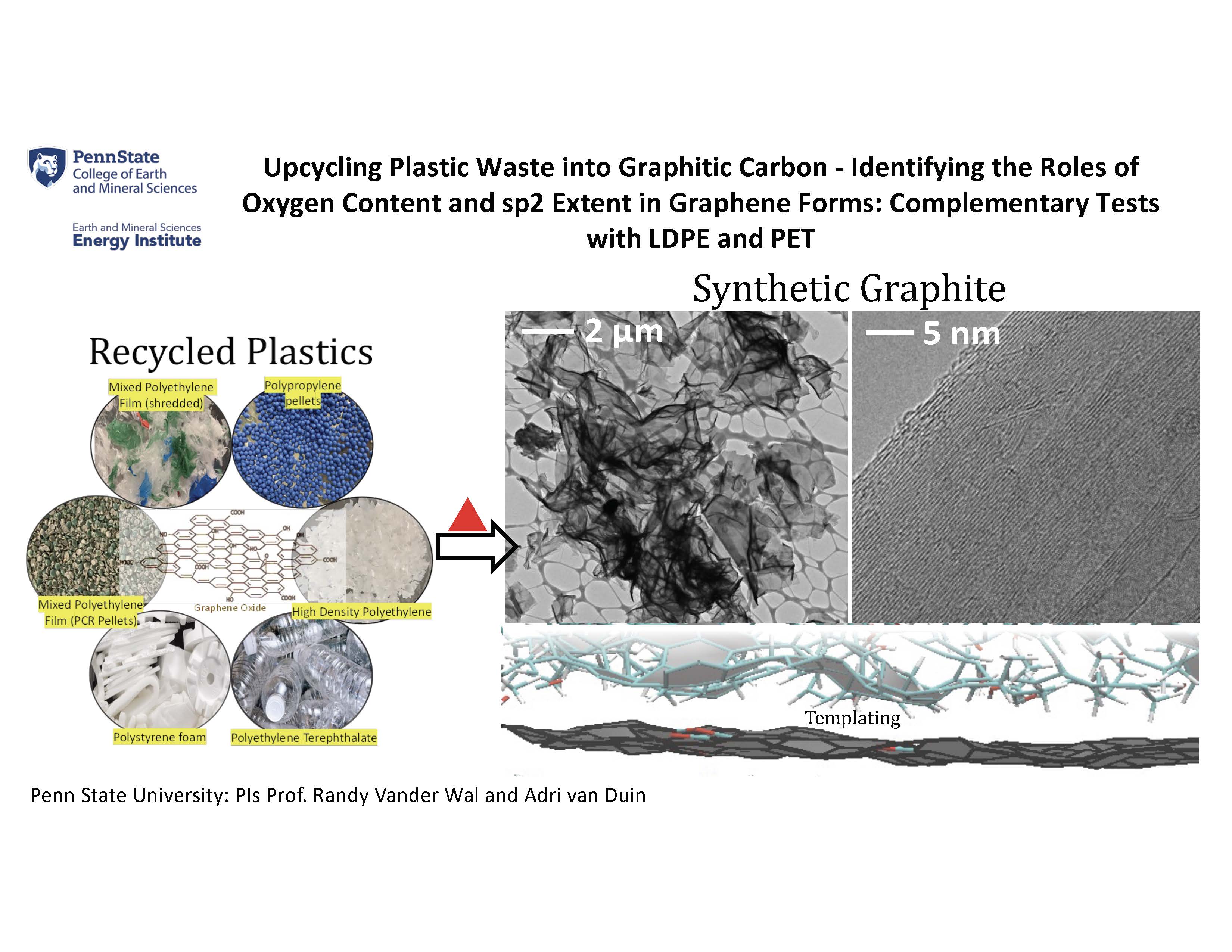
When heated, plastics typically crack into light gases through chain unzipping and β-bond scission. This translates into low carbon yield and non-graphitizable residual carbon. We propose an innovative approach of employing GO as templating agent and closed reactor carbonization under autogenic pressure to increase yield and graphitic quality of carbons. Oxygen functional groups on GO would provide the necessary stabilization while the sp2 framework would serve as a template guiding reconstruction of polymer chains into graphitic material. The latter postulated templating effect could realize significant cost and energy savings by enabling graphitic structure at lower temperatures. Notably, a portion of this graphitic material could be used to derive the GO for templated graphitization of fresh feedstock, thus contributing to circular process and upcycling plastic economics. Upcycling overabundant plastic waste into graphitic carbons will reduce pollution, cost and CO2 emissions. Four commercially recycled plastics and their composites with GO have been used to evaluate comparative yield and graphitic quality of the obtained carbon materials. The key advantage of GO as additive is that it nets a substantial increase in carbon yields, nearly 300% in some plastics. GO/plastic composites possess lattice parameters comparable to graphitized anthracene coke.
Pure plastics have extremely low carbon yields as they go through chain unzipping and β-bond scission when heated to produce light gases. The stabilization provided by GO additive in a sealed reactor remarkably improves the solid carbon yield for all plastics and as much as ~250% for PE. Analysis of the yield improvement with different GO additives provided insights into the mechanisms of interaction between plastic and GO during pyrolysis. GO improves the carbon yield of plastics by three main mechanisms: 1) radical combination reactions between polymer and GO radicals, 2) stabilization of polymer radicals by GO’s -network, and 3) diffusional confinement of polymer radicals. The visual illustrations of these mechanisms are provided. These mechanisms can be influenced by the composition, (i.e., oxygen (%)), stacking height, and lateral size of GO, respectively. During carbonization, oxygen functional groups on GO gasify, leaving behind radicals on GO that undergo radical combination reactions with the surrounding polymer radicals. The MD simulations confirmed that the pyrolyzing polymer radical chains bond with the active sites on the GO and induce “healing” of the GO through ring formation. If the polymer radicals generated during carbonization are not stabilized, they continue to crack into smaller fragments to yield light gases and little solid carbon. The stabilization provided by GO’s -network can increase the lifetime of the polymer radicals, so that they can undergo aromatization and growth, rather than cracking. Notably, GO can also aid the alignment of these aromatics and subsequent ordered growth via - interactions. This interpretation of better structure and ordering through - interactions is supported by an improved RDF observed in MD simulations of the additive systems. During carbonization, smaller gaseous polymer radicals are generated, while large solid GO sheets stabilize them and confine their movement. This promotes inter-radical reactions, leading to cyclization, aromatization, and subsequent concatenation reactions. The carbon yield improvement was also observed with graphene additive having no oxygen but a large lateral size, further supporting the importance of diffusional confinement and stabilization mechanisms. Indeed, the TGA-DSC analysis and MD simulations confirm that the embedded GO promotes endothermic reactions such as melting, chain unzipping, etc. in the initial lower temperature range and later leads to a higher extent of exothermic reactions associated with stable products. Aligned with TGA-DSC results, MD simulations showed that the polymer unzipping is accelerated through interaction with additives.
In addition to the remarkable yield increase, the crystallite size and nanostructure improve markedly for HDPE and PET. A trade-off between carbon yield and crystallite size was noted for other plastics, likely due to the mild crosslinking responsible for the observed yield increase. The cokes produced from all recycled plastics are highly graphitizable, with crystallite parameters comparable to the industrial graphitization standard – anthracene coke. The synthetic graphites exhibit thin flakes characterized by long continuous lamellae and a large stacking height. The particular blend of waste plastics (50% PE + 50% PS) forms a graphitic carbon with a uniform crystalline phase and slightly better crystallite sizes than the separated plastics. This phenomenon can be attributed to the synergistic interactions between the aliphatic structure of PE and the aromatic framework of PS, a well-established concept in the industrial production of graphites from pitches.
After graphitization, all the heteroatoms leave and remarkably high purity (~100% carbon) synthetic graphite was obtained. This was validated by X-ray photoelectron spectroscopy and TGA oxidation analysis, as detailed in the supplementary information. This study demonstrates the versatility of recycled plastics in various forms, including flake, reprocessed pellets, foams, colored variants, and even mixed compositions, as viable feedstocks for obtaining uniform crystalline-phase graphitic carbon. Such versatility holds significant importance, as it addresses major challenges in plastic recycling, specifically the issues of separation and transportation. More broadly this work outlines a solution intersecting three challenges – 1) declining availability of graphitic carbon precursors, 2) projected supply shortfall driven by rapidly rising graphite demand for batteries, and 3) growing abundance of plastic waste. The approach reduces plastic waste and the CO2 footprint associated with the manufacture of graphitic carbons by displacing mining for natural graphite or oil recovery and refining for aromatic feedstocks. Beyond their application in batteries, these waste plastic-derived carbons can be used as polymer additives for improving mechanical properties, conductivity, abrasion resistance, increased protection from UV, pigmentation, etc. Transforming excess plastic waste into graphitic carbons can play a pivotal role in advancing the shift towards renewable energy sources, simultaneously mitigating pollution, lowering costs, and curbing CO2 emissions.
In this initial study, six commercially recycled plastics and blends with GO were evaluated for their comparative carbon yield and graphitic quality. GO provided excellent stabilization through the radical sites formed by the leaving groups. The key advantage of GO as an additive is that it netted a substantial increase in carbon yields, nearly 250% in some plastics. In addition to the significant increase in carbon yield, the addition of GO enhanced crystallite size and graphitic quality for HDPE and PS. Across all recycled plastics, the carbons exhibited remarkable graphitizability, featuring crystallite parameters on par with those observed in the model graphitizable material, namely anthracene coke. The synthetic graphite derived from waste plastic exhibited thin flakes with well-stacked, long, and continuous lamellae. These features are advantageous to anode material in LIBs. The interaction mechanisms between graphenic additives and waste plastics leading to improved yield and quality of graphitic carbon were discovered through collaborative experimental investigations and atomistic-scale molecular dynamic simulations. This demonstrated production of high-value carbons from consumer single-use waste plastics will boost recycling and enable other related uses for carbon materials formed from plastic waste.
Acknowledgements
This material is based upon work supported by the National Science Foundation under Grant CBET-2309333
Reference
Gharpure, A., Kowalik, M., Vander Wal, R. L., & van Duin, A. C. (2024). Upcycling Plastic Waste into Graphite Using Graphenic Additives for Energy Storage: Yield, Graphitic Quality, and Interaction Mechanisms via Experimentation and Molecular Dynamics. ACS Sustainable Chemistry & Engineering.