2025 AIChE Annual Meeting
(490a) Continuous CO Synthesis from the Air at Ambient Temperature and Pressure by an Integrated Direct-Air-Capture and Direct-Carbonate-Reduction System
Authors
DAC is one of the highly promising approaches to reduce atmospheric CO2. However, conventional DAC techniques using CO2 absorbers require high temperatures (>800˚C) to release the absorbed CO2 gas [2] that will be converted to valuable chemical species in the subsequent process. Therefore, it would be difficult to drive these DAC devices, requiring frequent start-stop operations, by only time-fluctuating renewable energies. DCR could eliminate the high-temperature process for DAC by directly reducing carbonates in the liquid CO2 absorber supplied from the DAC devices using electrochemical means. Furthermore, DCR operates at ambient temperature and pressure, in contrast to thermocatalytic CO2 reduction processes that require high temperatures and pressures. However, most of previous DCR studies have used electrolytes with carbonate concentrations significantly higher than those at atmospheric equilibrium [3]. To address this, a DAC-DCR system has been reported to adopt a batch process to increase the carbonate concentration in the electrolyte to be equilibrated with the air over an extended duration [4]. However, the batch process requires switching operations for each batch, making the system more complex. In the present study, we have devised an innovative method, which enables the circulation of a CO2-absorbing electrolyte between the DAC and DCR units, capturing CO2 from the air and converting it into CO via electrochemical carbonate/bicarbonate reduction, thus realizing continuous operation at ambient temperature and pressure.
The DAC unit consists of a gas-liquid contactor, pumps, and CO2 gas meters. The CO2-absorbing electrolyte of a mixed aqueous solution of potassium carbonate (K2CO3) and potassium bicarbonate (KHCO3) is sprayed into the gas-liquid contactor where air flow is supplied simultaneously. The CO2 capture rate is determined from the difference between the CO2 concentrations at the intake and exhaust of the air flow. A part of the electrolyte ejected from the gas-liquid contactor is transferred to the DCR unit, while the remainder is recirculated within the DAC unit. The DCR unit, composed of a carbonate-reducing electrolyzer, a power supply, pumps, and a CO/CO2 gas meter, reduces the carbonate ion to CO gas. The electrolyzer assembly includes a nanoporous gold cathode formed by etching a gold-silver alloy, a Nafion™ 117 film solid electrolyte, and a platinum mesh anode. The CO concentration measured at the exhaust of the electrolyzer using a gas analyzer is converted to the CO synthesis rate.
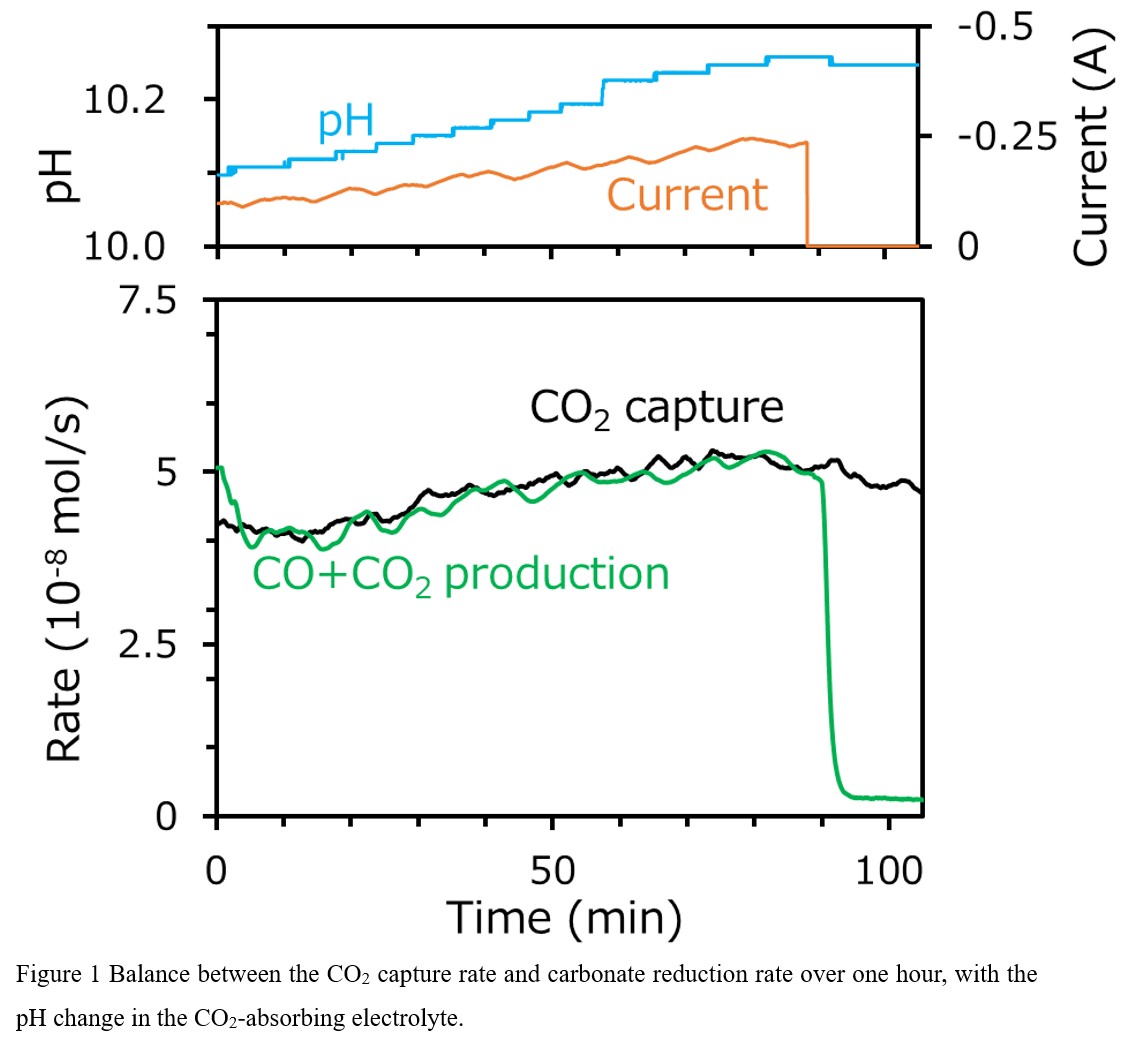
To realize our concept of the continuous operation using the DAC-DCR system at a constant CO synthesis rate, it is crucial to balance the CO2 capture rate and the CO synthesis rate. Therefore, both rates were monitored in real time with a time resolution of 1 s, and the CO synthesis rate was fine-controlled throughout the operation. As an initial state, the composition of the CO2-absorbing electrolyte was adjusted to be close to that at atmospheric equilibration, leading to a CO2 capture rate of 4×10-8 mol/s. The CO synthesis rate was controlled by changing the current supplied to the DCR electrolyzer to match the CO2 capture rate. This control successfully balanced the two rates over 1 h, as is clear from Fig. 1, showing continuous CO synthesis from CO2 in the air. However, both rates slightly increased during the operation. One possibility for this is that the current control was insufficient to handle the slow response of the DAC-DCR integration. If the excess current is supplied to the DCR electrolyzer, the carbonate concentration in the CO2-capturing electrolyte decreases with an increase in pH, which in turn further increases the CO2 capture rate. An improvement of the current control makes it possible to balance the CO2 capture rate and the CO synthesis rate with the desired CO production rate constant maintained over a long duration.
In conclusion, we demonstrated continuous and stable operation of the newly developed DAC-DCR system at ambient temperature and pressure, in contrast to conventional DAC and CO2 reduction devices that require high temperatures and high pressure. This great feature enables intermittent operation, making the system suitable to be driven by time-fluctuating renewable energies including solar power.
References
[1] Y. Sakamoto, Y. Nishimura, Y. Mizutani, S. Mizuno, R. Hishinuma, K. Okamura, Y. Takeda, M. Iwasaki, “Continuous CO synthesis from ambient air by integrating direct air capture and direct carbonate reduction using an alkaline CO2-absorbing electrolyte operating at room temperature”, Carbon Capture Sci. Technol., 12 (2024) 100225.
[2] D. W. Keith, G. Holmes, D. St. Angelo, K. Heidel, “A process for capturing CO2 from the atmosphere”, Joule, 2 (2018) 1573-1594.
[3] Y. C. Xiao, C. M. Gabardo, S. Liu, G. Lee, Y. Zhao, C. P. O’Brien, R. K. Miao, Y. Xu, J. P. Edwards, M. Fan, J. E. Huang, J. Li, P. Papangelakis, T. Alkayyali, A. S. Rasouli, J. Zhang, E. H. Sargent, D. Sinton, “Direct carbonate electrolysis into pure syngas”, EES Catal., 1 (2023) 54-61.
[4] O. Gutiérrez-Sánchez, B. de Mot, N. Daems, M. Bulut, J. Vaes, D. Pant, T. Breugelmans, “Electrochemical conversion of CO2 from direct air capture solutions”, Energy Fuels, 36 (2022), 13115-13123.