2025 AIChE Annual Meeting
(53f) Computational Insights into Hydrodehalogenation Trends on Rhodium Catalysts
Authors
MD Shahriar Hossain - Presenter, University of Houston
Fahmida Akter, University of Houston
Lauren Babb, University of Maine
Dale Green, University of Houston
Rachel Austin, Barnard College
Lars Grabow, University of Houston
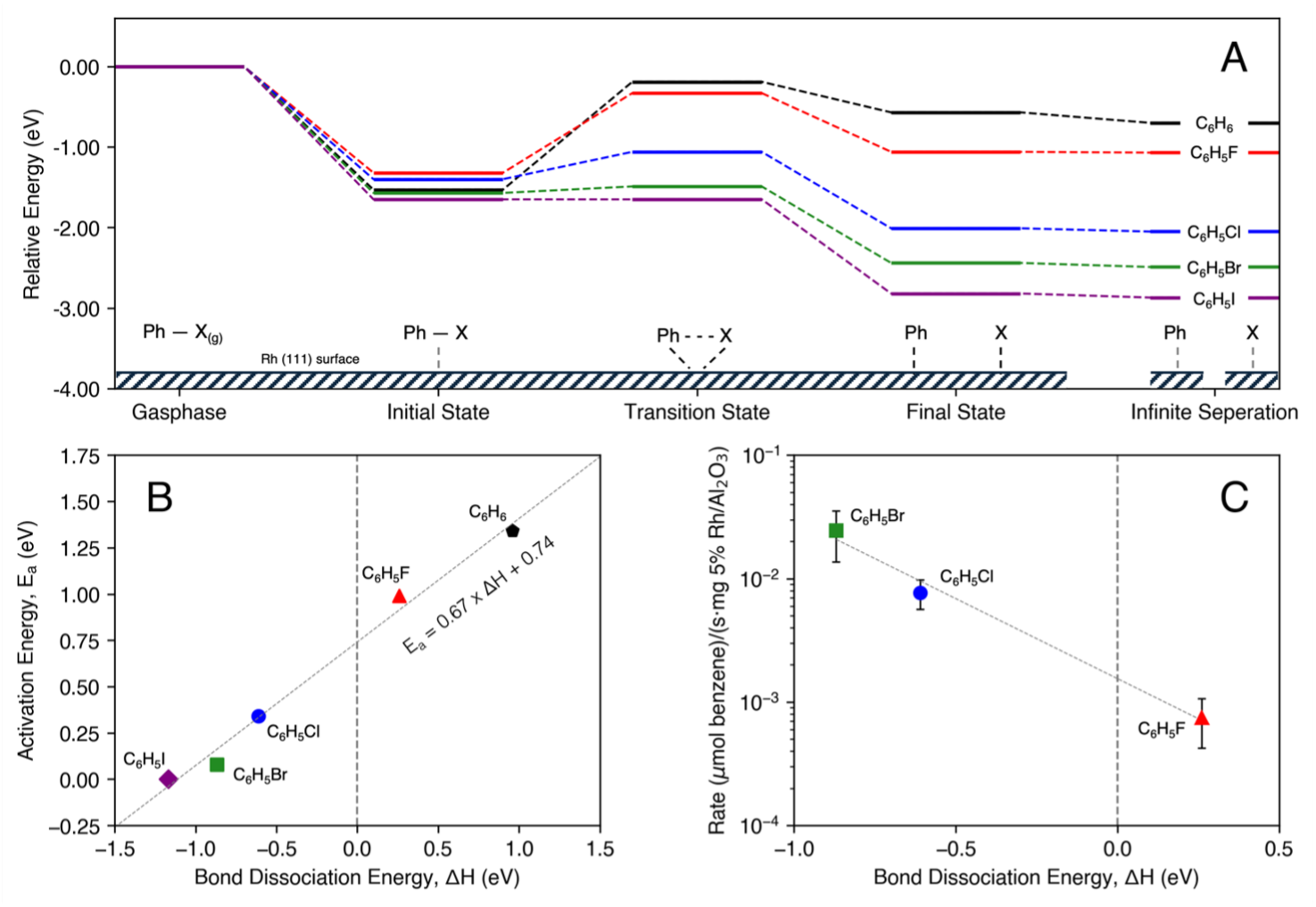
Selective cleavage of carbon–halogen (C–X) bonds is crucial for the remediation of halogenated organic pollutants, which are persistent byproducts of industrial activity. Supported rhodium catalysts, such as Rh/Al2O3, offer a promising platform for hydrodehalogenation (HDH) in aqueous environments using hydrogen as a clean reductant. Experimental studies demonstrate that Rh/Al2O3 can effectively cleave C–X bonds in halobenzenes under mild conditions, with reaction rates correlating to the C–X bond dissociation energy. X-ray photoelectron spectroscopy (XPS) confirms that the catalytically active species is metallic Rh, and deactivation relates to halide accumulation on the surface. To gain insight into the catalytic mechanism, density functional theory (DFT) calculations and microkinetic modeling were utilized to study PhX (X = F, Cl, Br, I) on Rh(111). These studies reveal that halobenzene adsorption is strongest at low coverage in flat geometries, with activation barriers following a Brønsted-Evans-Polanyi relationship. Microkinetic models suggest a significant role in surface coverage effects, particularly for strongly binding species like fluorine, which influences surface speciation and reaction kinetics. These findings underscore the critical role of surface coverage in controlling activity and selectivity. Thus, a coverage-dependent phase diagram for Rh(111) mapping the most thermodynamically stable adsorbate configurations as a function of temperature, pressure, and halogen identity serves as a useful predictive tool in this context. This framework improves our understanding of competitive adsorption and guide the design of enhanced HDH catalysts for environmental detoxification by facilitating more accurate predictions of active surface states under varying reaction conditions. Ultimately, this work contributes to developing efficient catalytic pathways to mitigate halogenated pollutants in industrial and environmental settings.