2025 AIChE Annual Meeting
(54c) Comparisons of Light Alkane Oxidation over Zeolite and Carbon Catalysts Containing M-N4 Active Sites
Authors
Ethan Iaia - Presenter, The University of Alabama
Ganesh Rana, The University of Alabama
Tibor Szilvasi, University of Alabama
Martin G. Bakker, The University of Alabama
James Harris, University of Alabama
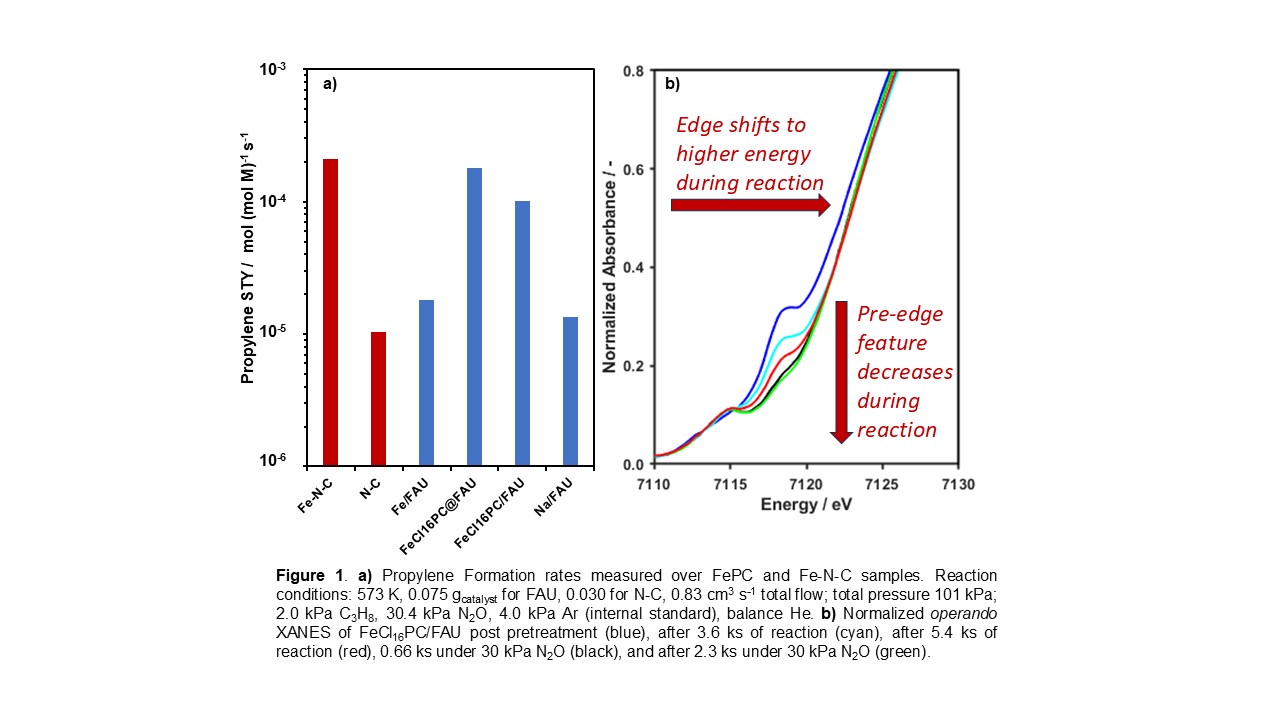
In 2021, DOE’s Office of Fossil Energy and Carbon Management released a report highlighting the need for new technologies capable of converting light alkanes that would normally be flared into value-added products. Thermocatalytic partial oxidation of alkanes is encumbered by combustion of weaker C-H bonds present in products. Metalloenzymes can limit overoxidation and improve selectivity to desired products, prompting researchers to investigate synthetic analogs. Many studies have focused on zeolites and metal organic frameworks, whose structures can be tuned to mimic metalloenzymes. However, these materials often contain heterogeneities in their metal species, which complicates catalyst characterization and the development of structure-function relationships. Square planar iron-containing molecular complexes, such as metal phthalocyanine (MPC), are known to catalyze alkane oxidation in the liquid phase, and contain similar Fe motifs to metalloenzymes and metal nitrogen-doped carbons (M-N-C) recently reported to be competent catalysts for alkane oxidation. In our previous work, occlusion of MPC within the supercages of faujasite zeolites (MPC@FAU) led to increased site time yields (STYs; per mol M) in gas phase CO oxidation relative to MPC dispersed on the exterior of FAU, or M-ion-exchanged FAU. Here we compare rates over MPC@FAU and M-N-C catalysts with varied metal binding sites in the oxidative dehydrogenation (ODH) of propane with N2O. M-N-C samples undergo initial deactivation while MPC@FAU samples do not. Propylene STYs are similar over M-N-C and MPC@FAU. Apparent activation energies (~100 kJ mol-1) are consistent with previous reports, and apparent reaction orders for both reactants were <1 for both types of samples. Operando XANES data suggest metal centers in both MPC@FAU and M-N-C are partially oxidized during reaction. Potential mechanisms leading to observed apparent kinetics are discussed. With these results, we aim to demonstrate the effectiveness of studying well-defined MPC@FAU materials as models for the less-uniform metal active sites of M-N-Cs.