2025 AIChE Annual Meeting
(536c) Combined Roles of Electrokinetic and Stress-Driven Flow Mechanisms in Porous Anodic Oxide Formation
Author
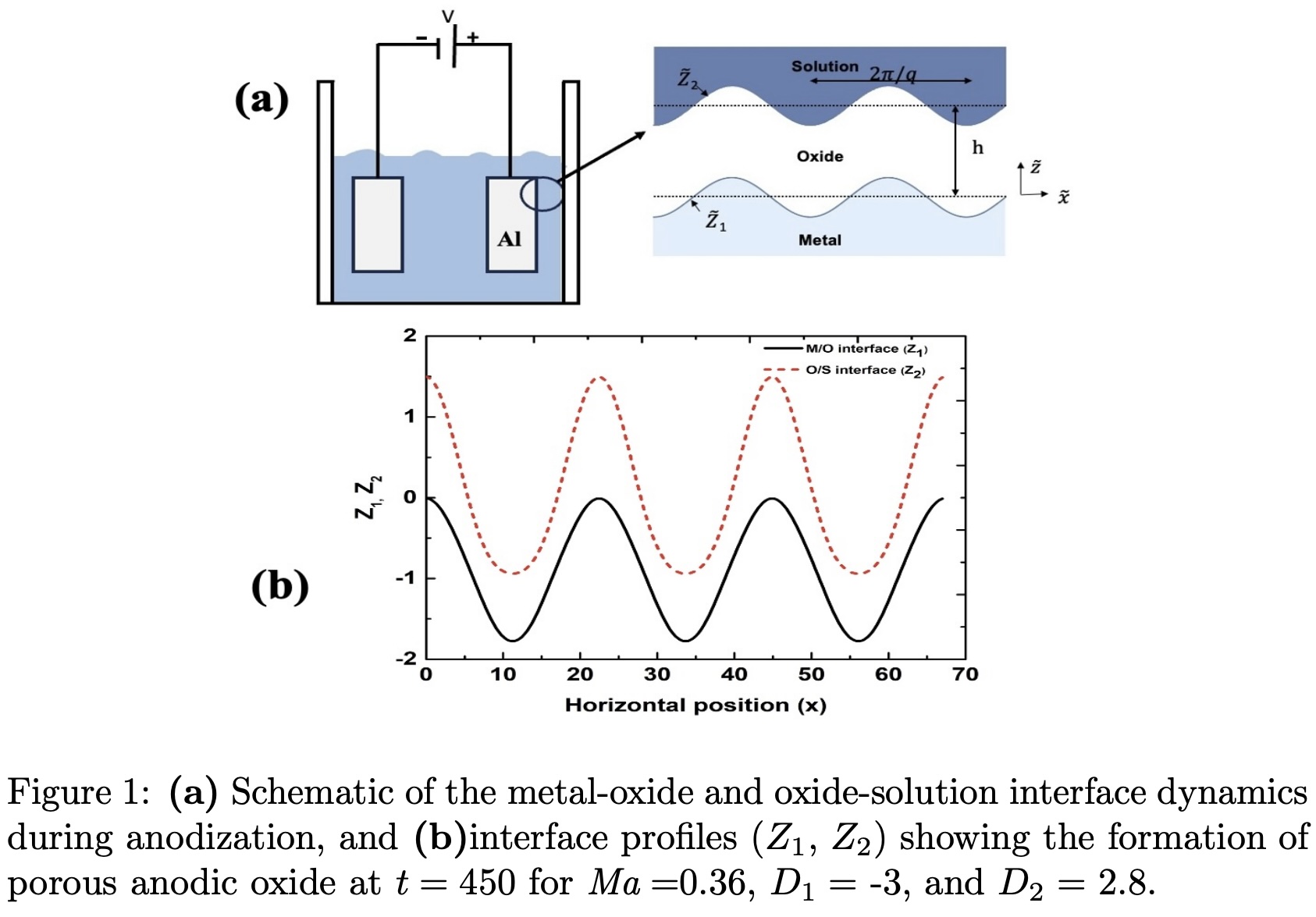
The anodization of aluminium in acidic electrolytes leads to the development of a nanoporous aluminium oxide layer. A combined model for the anodization process is developed to investigate the initiation and growth of pores in anodic oxides, incorporating both viscous oxide flow and electrokinetic dissolution mechanisms. A linear stability analysis reveals that the instability arises from the destabilizing effects of stress-driven oxide flow and field-assisted electrochemical reaction kinetics, while stability is maintained through the opposing influences of electrochemical oxide formation and surface energy. Notably, the analysis indicates that both stress-induced flow and electrokinetic mechanisms play significant roles in the early stages of pore development. Considering only electrokinetically driven instability leads to a substantial overestimation of pore spacing. The model is demonstrated to be well-posed and consistently predicts a long-wave instability across all parameter values. Furthermore, the evolution equations for the metal-oxide and oxide-solution interfaces are derived using the Weighted-Residual Integral Boundary Layer (WRIBL) method, which employs the long-wave approximation to simplify the governing dynamics. The WRIBL model predictions are in good agreement with the linear stability analysis of the long-wave equations. Numerical simulations reveal a subcritical nature of the instability, that drives pore initiation and deepening (cf. Figure 1), characterized by oxide flow from the pore base toward the walls, consistent with observations from earlier tracer experiments.observations of pore formation.