2025 AIChE Annual Meeting
(444d) A Combined Experimental-Computational Methodology to Accelerate Screening and Discovery of Solid Sorbents for Moisture-Swing Carbon Capture
Authors
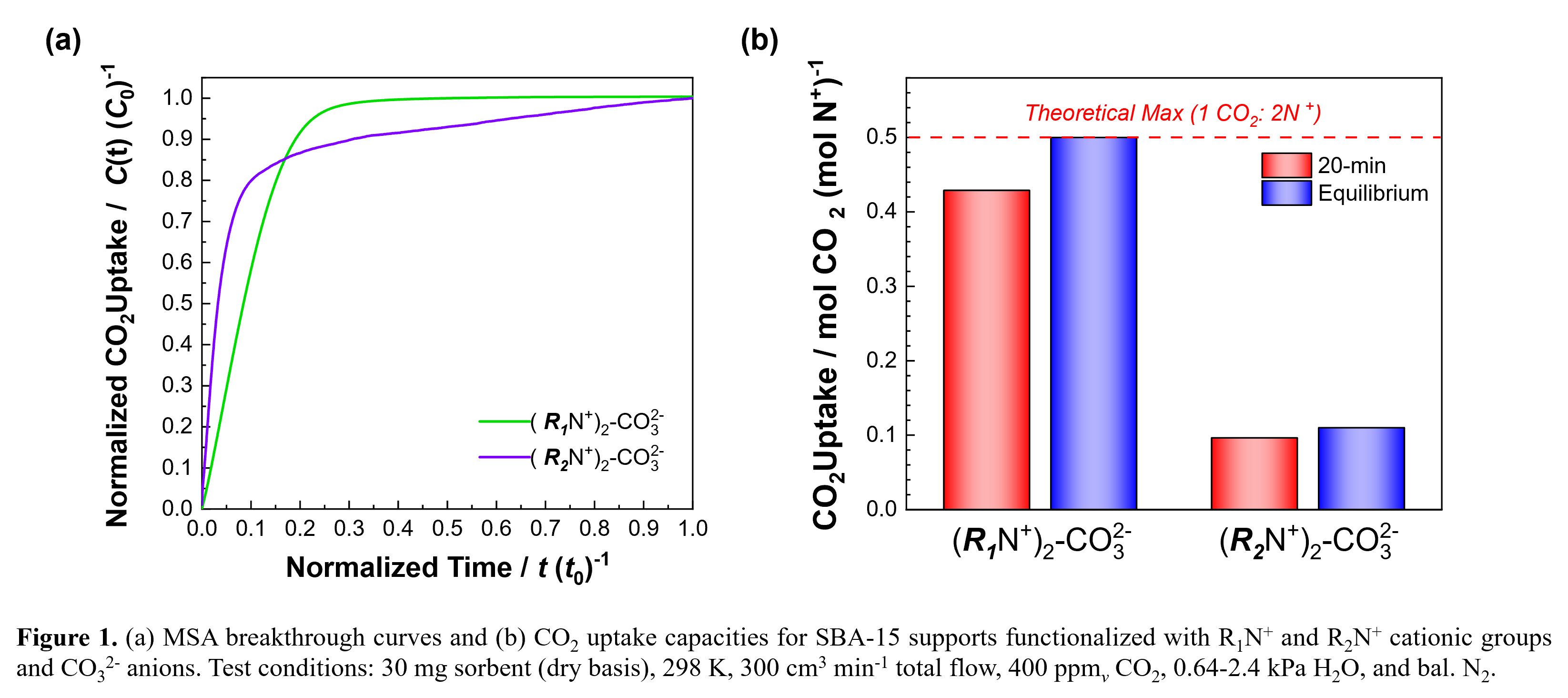
Here, we integrate computational modeling, synthesis, characterization, and MSA breakthrough analysis to design a series of ordered, mesoporous sorbents containing different cationic functional groups. A high-throughput molecular modeling workflow was used to identify target cation-anion pairs by: (i) calculating free energies for OH- formation as a function of active site hydration, and (ii) predicting MSA reactivity based on a shift in the CO2 capture step from exergonic to endergonic as a function of humidity. Computationally predicted model sorbents were prepared by functionalizing SBA-15 mesoporous silica with quaternary ammonium cations containing organic substituents of varying chain length (e.g., R1, R2) charge-balanced by carbonate anions. The sorbents were evaluated for moisture-responsive CO2 capture over multiple cycles in a fixed bed adsorber at 298 K with 400 ppmv CO2 and between 0.64-2.4 kPa H2O. Our findings reveal that cation-anion interactions can be influenced by changing the organic substituents, leading to an ~5× increase in CO2 uptake (normalized per mole of N+) during a 20-min adsorption step without adversely affecting sorption kinetics (Figure 1). This approach demonstrates that molecular modeling in conjunction with empirical relationships between material properties and performance can enable pre-screening of potential MSA sorbents and guide the discovery of new materials for CO2 capture.
References:
[1] T. Wang, K.S. Lackner, A. Wright, Moisture Swing Sorbent for Carbon Dioxide Capture from Ambient Air, Environmental Science & Technology, 45 (2011) 6670-6675.
[2] S. McCord, A.V. Zaragoza, V. Sick, Y. Yuan, A. Spiteri, B.A. McCool, R.R. Chance, Life Cycle Analysis of a Hybrid Direct Air Capture System Enabling Combined CO2 and Water Extraction from Ambient Air, Carbon Capture Sci. Technol. 15 (2025) 100403.
[3] W.S. Wang, X.X. Zhang, J. Liu, C.Y. Liang, J.Z. Niu, F.Y. Wang, Review of moisture swing sorbents for carbon dioxide capture from ambient air, International Journal of Global Warming, 32 (2024).