2025 AIChE Annual Meeting
(575d) CO2 Adsorption and Plasma Induced Desorption Behavior of Ca-Based Layered Double Hydroxides
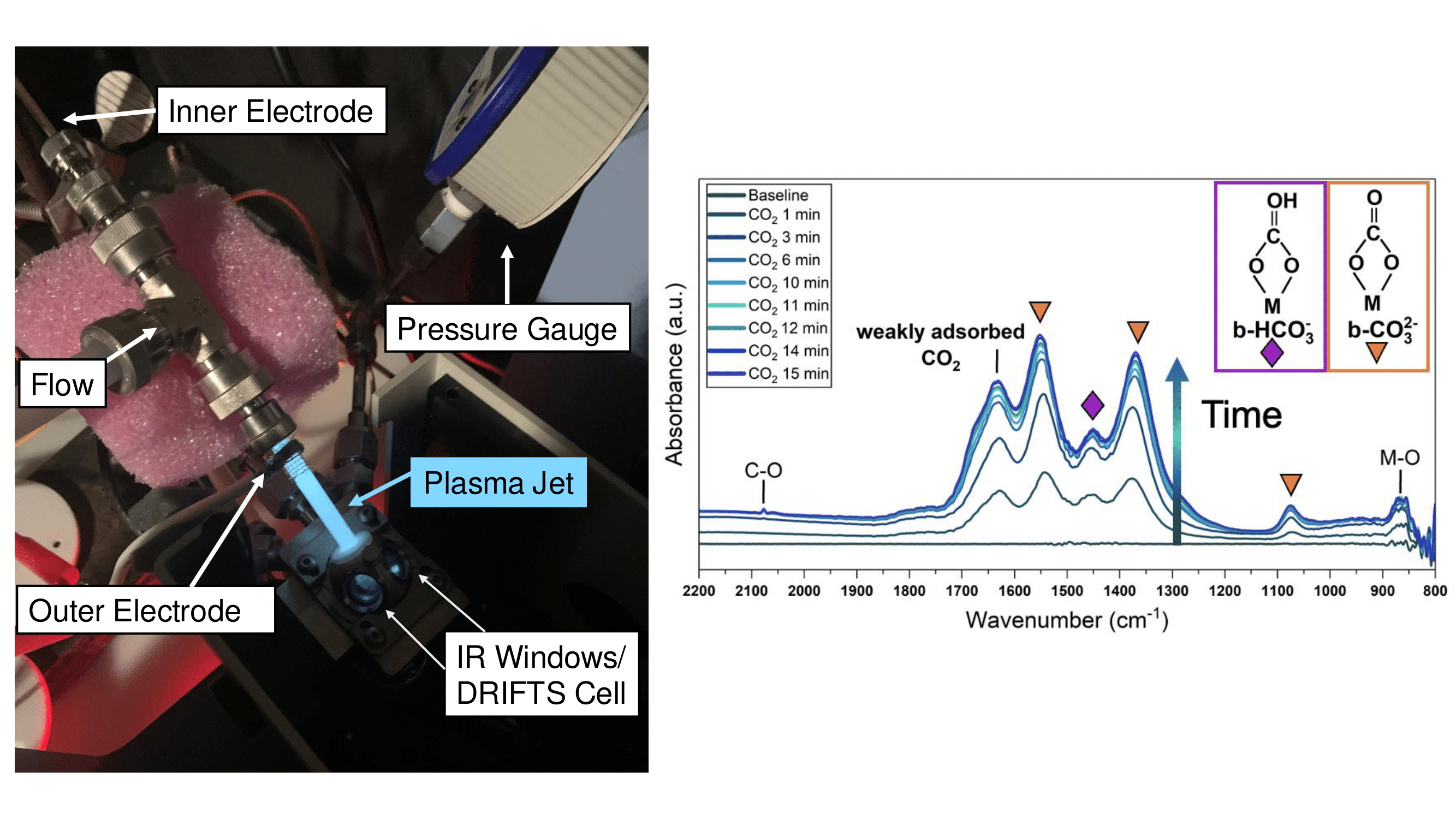
Layered double hydroxides (LDHs) are a class of solid adsorbents characterized by a bimetallic hydroxide layer intercalated with water and anions. Calcium-based LDHs have shown recent promise to compete with other solid adsorbents for CO2 capture, with a reported capture capacity of 4.3 ± 0.5 mmol/g at 30 °C and 40% relative humidity. In particular, LDH CO2 capture capacity improves in the presence of water, while for adsorbents such as MOFs and zeolites H2O and CO2 typically compete for adsorption sites. Although calcium-based LDHs show promise for concentrated CO2 sources of high humidity, such as flue gas (5-15 vol. % H2O), the specific mechanism of adsorption is incomplete including the role of H2O molecules. Moreover, the application of non-conventional regeneration approaches for Ca-based LDHs remains largely unexplored. In this work, the mechanism of CO2 adsorption by chloride-containing Ca-based LDHs with different trivalent cations will be discussed together with desorption behavior caused by exposure to low-temperature non-equilibrium plasma. Adsorption and desorption behavior have been elucidated using in operando diffuse reflectance infrared Fourier transform spectroscopy coupled with a residual gas analyzer, with complementary material characterization via X-ray diffraction, thermogravimetric analysis, and X-ray photoelectron spectroscopy. Initial experiments have been performed on the impact of humidity on CO2 adsorption, with in operando thermal desorption being carried out up to ~650 °C (limit of in operando conditions). These experiments show the unique role of interlayer H2O in the LDH for conversion of CO2 to carbonate species during adsorption and that CO2 desorption commences at a lower temperature than currently reported in the literature. Moreover, CO2 desorption experiments conducted using low-temperature non-equilibrium plasma reveal that desorption is aided by the application of the plasma, and some CO2 is found to desorb even at room temperature.