2025 AIChE Annual Meeting
(584bu) CO Dissociation on MgO Supported Ru Catalyst and the Effect of Al Oxide Dopant.
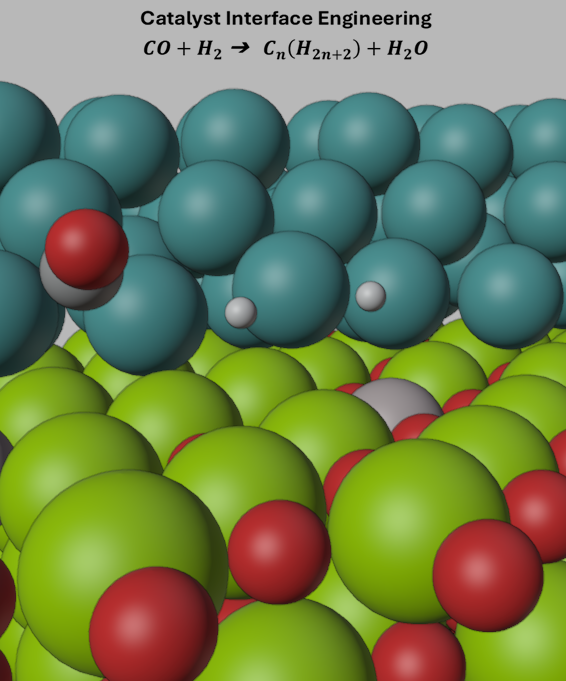
Fischer-Tropsch synthesis (FTS) is a well-known chemical process that transforms synthesis gas (CO and H₂) into longer hydrocarbons, critical for sustainable fuel production. Previous studies have suggested CO activation to be important step in Fischer-Tropsch synthesis. Here, we use density functional theory calculations to study the effects of aluminum doping of the MgO support on Ru/MgO catalysts. Employing a Ru nanorod supported on MgO (001), we examine CO activation, dissociation, and C–H bond formation at the Ru(111)/MgO interface and the effect of Al doping of the support on the active site. Although doping the support doesn’t affect the most favorable adsorption site for CO on Ru, the Al increases the electron density at the Ru active sites, enhancing charge transfer to the adsorbed CO. The Al incorporation also promotes oxygen vacancy formation that can affect CO dissociation and C–H bond formation. Bader charge analysis indicates a reduction in the carbon atom’s charge, consistent with the weakening and elongation of the C–O bond and the decrease in vibrational frequency. These electronic and structural changes suggest a more favorable CO activation on the Al-doped Ru/MgO catalyst. This study underscores the potential of tuning support-induced electronic interactions to optimize CO activation in catalysts relevant to Fischer-Tropsch synthesis.