2025 AIChE Annual Meeting
(660c) Chemical Engineering of Binding Sites in Porous Polymer Networks for the Selective Removal of PFAS and Selenium from Water
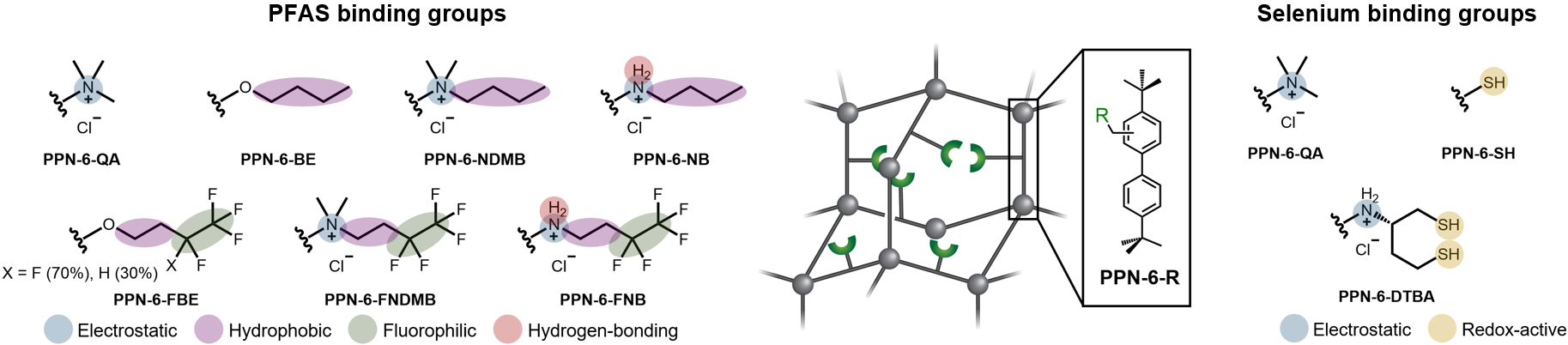
The high surface area, porosity, and structural robustness of PPNs make them ideal candidates as adsorbent media for use in water purification. Most importantly, the covalently linked monomers that make up the network can be appended with chemical functional groups. It is these functional groups that are responsible for the solute binding capabilities of the PPNs. Understanding target solute binding mechanisms is thus a prerequisite for the development of improved adsorption sites in not only PPNs but any adsorbent material.
PFAS are a class of synthetic organofluorine molecules that typically consist of a hydrophobic fluorinated alkyl “tail” and a hydrophilic “head”. Now found in nearly every water source on the planet, PFAS bioaccumulate in the human body and repeated exposure to even trace amounts can have serious health consequences. This has prompted significant regulatory action, and water treatment facilities are desperately trying to implement systems to meet the low allowable PFAS limits. Among treatment technologies, adsorbents are ideally suited for the efficient removal of PFAS, but currently suffer from low adsorption capacities and selectivities, and slow kinetics. When targeting PFAS uptake in adsorbent media, favorable interactions with both the polar head and non-polar tail of the molecule can be leveraged. While the PFAS head can engage in hydrogen-bonding and electrostatic interactions, the tail can participate in hydrophobic and fluorophilic interactions. To enable the design of next-generation PFAS adsorbents, the relative importance of these interactions must be elucidated.
To experimentally determine the ideal combination of binding interactions for PFAS adsorption, a representative porous polymer network (PPN-6) was post-synthetically appended with functional groups expected to engage in unique combinations of electrostatic, hydrogen-bonding, hydrophobic, and fluorophilic interactions with PFAS molecules. In total, seven different functionalized PPNs were produced and their PFAS adsorption performance was tested. Previous work in adsorbent design has primarily focused on the removal of legacy long-chain PFAS from simple water matrices, but we consider it imperative to fully evaluate and compare the performance of our materials in a variety of water compositions (i.e., deionized water, simulated wastewater, real wastewater, and real groundwater).
Batch adsorption experiments and computational studies revealed that electrostatic and hydrogen-bonding interactions drive short-chain PFAS adsorption, while hydrophobic and fluorophilic interactions improve long-chain PFAS adsorption. In complex water matrices, a combination of electrostatic and fluorophilic interactions led to the greatest total PFAS removal. The best-performing material, functionalized with a fluorinated alkylammonium (PPN-6-FNDMB), selectively adsorbs PFAS with high capacity (up to 4.0 mmol/g) and rapid kinetics (equilibrium reached in < 30 s). Furthermore, PPN-6-FNDMB outperforms several commercial adsorbents, achieving near-complete removal of 21 different PFAS from a groundwater sample collected at a US Air Force base. The PFAS could subsequently be desorbed from PPN-6-FNDMB, concentrating them by a factor of over 50 times. The recycled PPN-6-FNDMB could then be reused with minimal losses in long-chain PFAS adsorption capacity over four cycles. Not only does this work present some of the best performing PFAS adsorbents reported to date, but the elucidation of the relative importance of different binding interactions will help guide the development of next-generation PFAS adsorbents.
The success of our work on PFAS adsorption encouraged us to explore how similar principles of functional group design can be applied to other challenging aqueous separations. One such separation is the removal of selenium from contaminated waters. Although selenium is an essential nutrient, excess selenium ingestion can have serious impacts on health. Similarly, elevated amounts of selenium in the environment can negatively impact aquatic and terrestrial wildlife. While low concentrations of selenium end up in water via natural erosion processes, certain industrial processes generate wastewater with dangerously high selenium concentrations. These industries have faced strict water discharge regulations, and consequently are continuously searching for new technologies that will increase the efficiency of selenium removal. Current selenium removal technologies include using zero-valent iron or bioreactors to reduce the selenium to insoluble forms. However, these technologies have low capacities and selectivities, slow kinetics, and generate large volumes of contaminated sludge that require further treatment and disposal. Once again, functionalized adsorbent media offer a promising alternative.
Present in the +4 (selenite, HSeO3−/SeO32−) and +6 (selenate, SeO42−) oxidation states, selenium oxyanions can engage in both electrostatic and redox-active binding interactions with adsorbent functional groups. Similar to the approach taken for PFAS-selective PPNs, we prepared three functionalized PPNs, with the goals of (i) elucidating the relative importance of these interactions and (ii) determining if a synergistic combination of interactions could lead to enhanced selenium removal. Through batch adsorption experiments in water samples spiked with selenium, we found that electrostatic interactions lead to rapid adsorption kinetics (equilibrium reached in <30 s). Meanwhile, redox-active interactions lead to the highly selective adsorption of selenite over competing ions in a simulated flue-gas desulfurization wastewater. Supporting our hypothesis of synergistic binding effects, the PPN containing functional groups capable of both electrostatic and redox-active interactions (PPN-6-DTBA) permitted the fast and selective adsorption of selenium from complex water matrices. The adsorption mechanism was investigated using x-ray spectroscopies, crystalline molecular analogs, and computational studies. Once again, this work not only presents one of the best selenium adsorbents reported to date, but it also exemplifies how careful chemical design of adsorbent binding sites can enhance their target solute removal performance.