2025 AIChE Annual Meeting
(343d) Charge-Based Sensing Platforms for Exosome Diagnostics
Authors
However, precision EV diagnostics still face two major challenges: low abundance (fM to pM) that requires high sensitivity and interference from dispersed proteins that yields false positives. We propose a charge-based sensing approach to overcome the specificity issue due to interference. As most interfering proteins are weakly charged, they do not produce a charge signal. In addition, we design antibody-conjugated silica nanoparticle (SiNP) charge reporters of the optimum size, such that the undocked reporters can be easily removed with a controlled washing step. This enhanced specificity improves the signal-to-noise ratio so that EV diagnostics can be conducted with untreated plasma. We address the sensitivity issue for this charge-based sensing strategy with an ion-depletion module based on permselective membranes (Slouka, Z. et al. Annu. Rev. Anal. Chem. 7: 317-335(2014)). Due to unidirectional counterion flux through the membrane, ionic strength polarization occurs across the membrane such that the sensing region is at DI condition (Debye length of a few hundred nanometers), compared with the bulk buffer of untreated plasma with an ionic strength that is 6 logs higher (< 1 nm Debye length). This allows the 50 nm SiNP reporters on top of the ~100 nm EV to be detected.
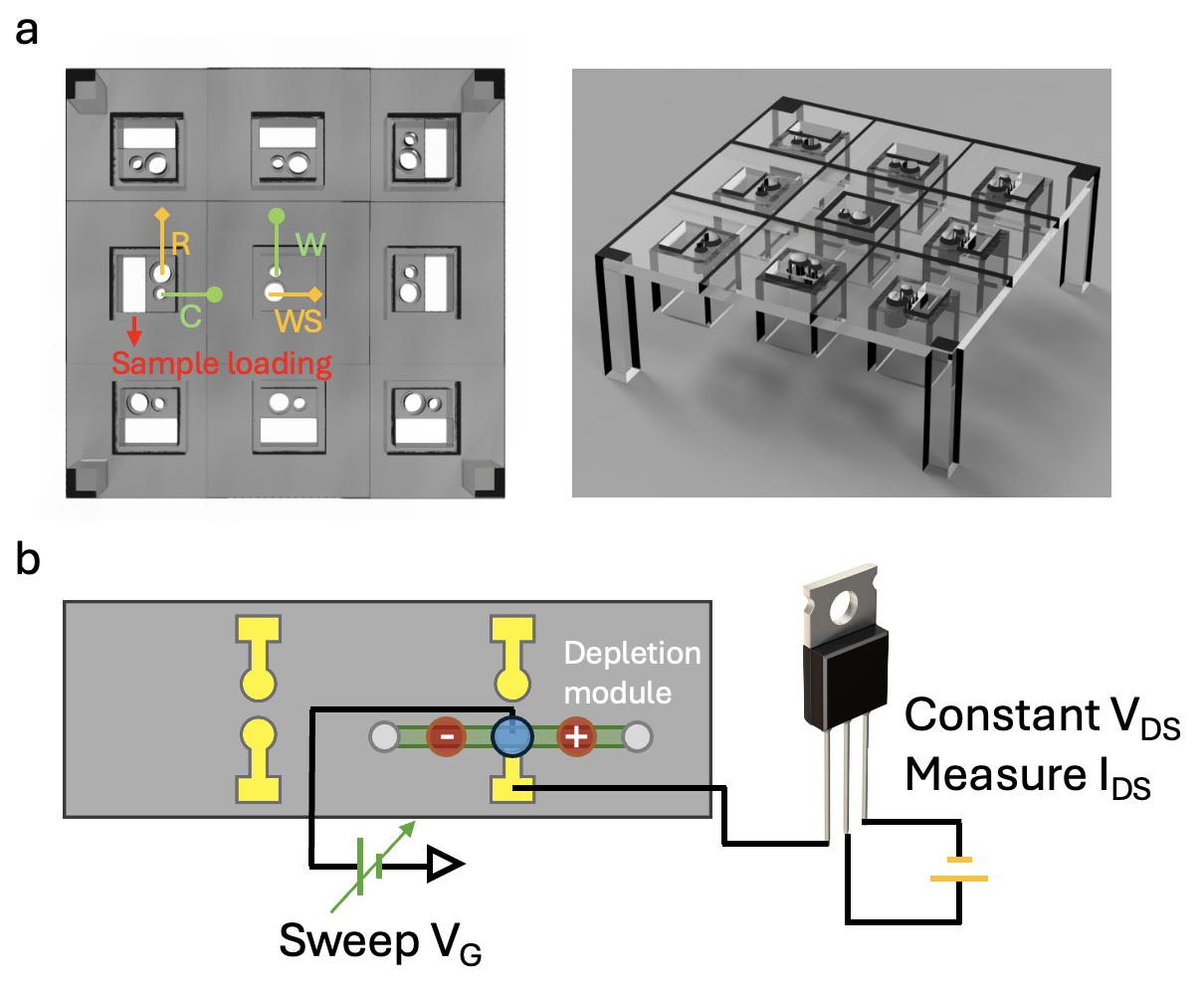
Two charge-based EV diagnostic technologies will be presented. One is a proteomic EV array based on membrane sensors, shown in Figure(a), that uses the same depletion membrane module as a sensor. With ion depletion, the bound SiNP reporters can effectively gate the ion current to produce a large signal in the "over-limiting" current region, as their charge and size can significantly affect the electroconvective instability responsible for the over-limiting current (Sensale, S. et al. The Journal of Physical Chemistry B 125: 1906-1915(2021)). A limit of detection of about 10 fM can be achieved. We have successfully detected multiple colocalized sEV markers such as CD99/NGFR for Ewing Sarcoma and MUCI/ITGβ3 for Ovarian cancer, in a cohort of 20 patients using this multiplexed array.
Our second charge-based EV technology is an integration of the field-effect transistor (FET) sensor with the ion-depletion module. Instead of using graphene or other expensive 2D semiconductors, we show that, with the depletion module, the sensitivity of a simple and inexpensive commercial extended-gate FET, as shown in Figure(b), can be improved 3 logs to yield a sub-fM limit of detection. As micron-sized FET sensors can be fabricated in massive quantities, this integration of ionic and electronic conducting materials promises an EV diagnostic platform for a massively large number of biomarkers. The extension includes a miRNA FET array downstream of the proteomic EV array to profile exosomal miRNAs (Ramshani, Z. et al. Commun. Biol. 2: 189(2019)) for specific EVs.