2025 AIChE Annual Meeting
(360d) Ce-Doped NiO Aerogel with Hierarchical Porosity and Abundant Oxygen Vacancies for Enhanced CO2 Cycloaddition to Epoxides
Metal–organic frameworks (MOFs) and zeolitic imidazolate frameworks (ZIFs) have been widely investigated as heterogeneous catalysts for the cycloaddition of CO₂ to epoxides due to their large surface area, tunable pore structures, and the presence of accessible Lewis acid and base sites. However, MOFs and ZIFs often exhibit limited hydrothermal and chemical stability, particularly under humid or high-temperature conditions, which can result in framework degradation and reduced recyclability. Furthermore, their intrinsic microporous structures can hinder the diffusion of bulky substrates, thereby lowering catalytic yields in reactions involving sterically demanding molecules. In contrast, metal oxides such as ZnO, MgO, and Nb₂O₅ offer excellent thermal and hydrolytic stability and can be readily regenerated, addressing the stability concerns associated with MOFs and ZIFs. Nonetheless, their relatively low surface area and limited CO₂ activation capacity typically result in reduced catalytic activity.
Consequently, various approaches, such as surface modification, elemental doping, single-atom catalysts (SACs), and crystal facet optimization, have been explored to improve the catalytic performances of CO₂ cycloaddition with epoxide by tailoring the surface characteristics of metal oxides. Liu et al. developed a heterostructured Fe₂O₃/NiFe₂O₄ catalyst via interfacial engineering, where electronic coupling and oxygen vacancies at the interface enhance Lewis acid–base site formation and boost CO₂ cycloaddition activity. Under relatively mild conditions (90°C, 0.4 MPa CO₂ and 0.36 mmol), the catalyst achieved 89.1% styrene oxide conversion and excellent recyclability. Choudhary et al. enhanced the catalytic performance of metal oxides by atomically dispersing Co2+ ions on a ZrO₂ support via a coprecipitation method. The resulting Co/ZrO₂ single atom catalyst achieved 100% conversion of epoxides to cyclic carbonates under solvent-free conditions (80°C, 0.2 MPa CO₂, 0.06 mmol TBAB), showing superior activity and recyclability compared to undoped and impregnated ZrO₂ catalysts. Wei et al. improved CO₂ cycloaddition efficiency by tuning ZnO (002) crystal facets to control surface oxygen vacancies and expose Zn sites. The resulting ZnO-Ar catalyst exhibited a 93.3% conversion under mild conditions (125°C, 2 MPa, CO₂, 5 mg catalyst), with outstanding recyclability and a synergistic mechanism between oxygen vacancies and surface Zn validated via DFT and spectroscopic methods.
A powerful approach for enhancing the catalytic performance of metal oxides is the synthesis of metal oxide-based aerogels. Aerogels are highly porous materials typically derived by supercritical drying of wet gels produced via sol-gel processes, effectively preserving their structural integrity. Utilizing metal oxide-based aerogels significantly overcomes the intrinsic limitation of low specific surface areas typically associated with conventional bulk metal oxides, thereby maximizing exposure of active catalytic sites. Moreover, the versatile nature of the sol-gel process allows facile incorporation and homogeneous dispersion of multiple metal ion precursors, readily yielding well-dispersed, multi-component metal oxide aerogels. Over the past decade, there has been considerable interest in employing these aerogels as catalysts, demonstrating their remarkable effectiveness across diverse catalytic reactions. Novak et al. applied a CeO₂-based aerogel with atomically dispersed Ni to the water–gas shift reaction. Compared to bulk counterparts, the aerogel catalyst showed markedly improved selectivity by suppressing methanation, owing to its high surface area and uniform metal dispersion. Kim et al. applied a fluorine-doped SnO₂ aerogel decorated with Pt nanoclusters to the hydrogen evolution reaction (HER). Compared to bulk Pt/C catalysts, the aerogel-based system showed significantly enhanced activity and long-term stability, owing to its high surface area, mesoporosity, and strong metal–support interaction. Furthermore, recent studies have expanded the scope of metal oxide aerogels to various catalytic applications, consistently showcasing substantial improvements in catalytic efficiency and stability. Despite these remarkable advancements, metal oxide-based aerogels have yet to be explored for the cycloaddition of CO₂ to epoxides.
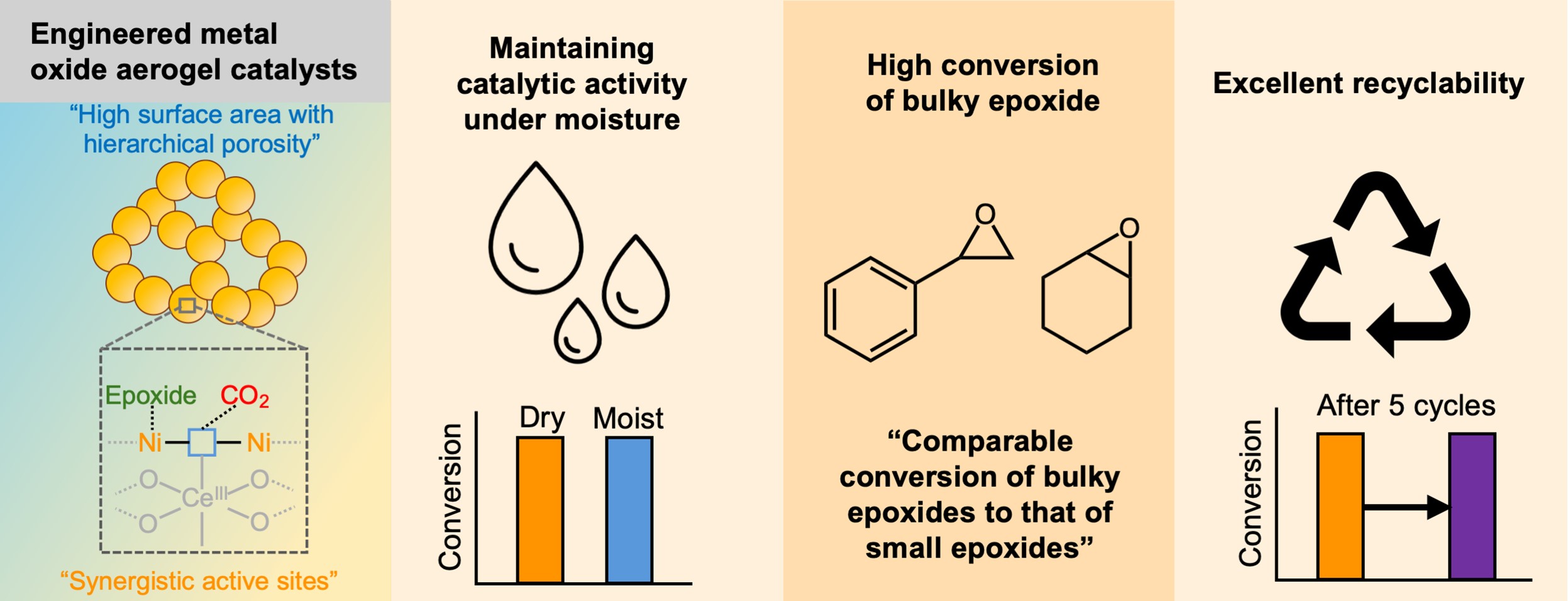
In this study, we successfully designed Ce-doped nickel oxide aerogels (NCA) via a facile sol-gel synthesis approach, incorporating 10 mol% Ce into NiO. The introduced Ce was uniformly dispersed within the NiO aerogel matrix, resulting in a highly hierarchical porous structure with a high BET surface area of 561 m2/g and pore volume of 3.3 cm3/g, significantly improved compared to conventional metal oxide materials. Furthermore, owing to the mild sol-gel conditions and supercritical CO₂ drying process, the synthesized aerogel exhibited a notably oxygen vacancy-rich and amorphous structure. Leveraging these unique structural properties, the NCA demonstrated remarkable catalytic activity for CO2 cycloaddition with various epoxides. Specifically, employing only 10 mg of catalyst under mild reaction conditions (80°C, 0.1 MPa, and 4 h) with a minimal amount of co-catalyst (0.02 mmol of TBAB), a notably high styrene oxide conversion of 98.0% was achieved. Importantly, the catalytic performance remained stable even under industrially relevant conditions containing 20 mol% moisture, maintaining an impressive conversion rate of 96.4%. Additionally, excellent recyclability was confirmed, as the catalyst maintained over 97% conversion efficiency after five consecutive reuse cycles, demonstrating negligible activity loss (<1%). Notably, this catalyst also efficiently converted bulky substrates such as cyclohexane oxide, achieving a remarkable 98.3% conversion within just 4 h, overcoming a longstanding challenge faced in recent CO₂ cycloaddition research. Our findings clearly demonstrate the effectiveness of metal oxide-based aerogels as catalysts for CO₂ cycloaddition, highlighting their significant potential to address industrial challenges related to moisture tolerance and cost efficiency. Thus, metal oxide aerogels represent a promising approach for practical and sustainable CO₂ utilization applications.