2025 AIChE Annual Meeting
(402ak) Caustic Aqueous Phase Electrochemical Reforming (CAPER) of Potassium Formate for Continuous Hydrogen Production
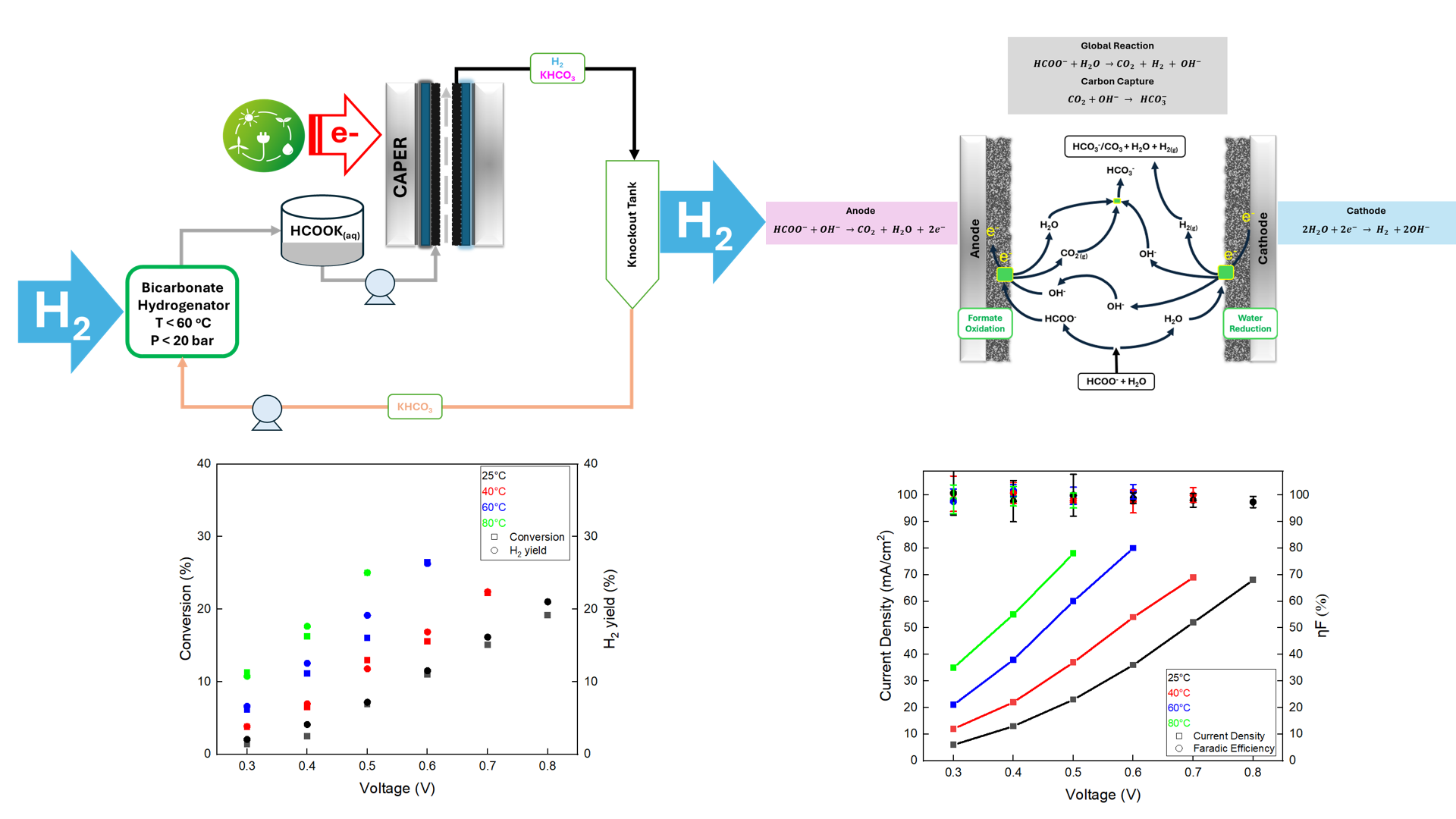
Despite being the most suitable alternative to current energy technologies, the intermittent nature of renewable energy from solar, wind, water, geothermal and biomass is a constraint in the energy transition. This constraint strongly demands the development of sustainable energy storage devices. Significant attention has been paid to hydrogen as a possible renewable energy carrier; however, hydrogen storage requires compression, liquefication and large volumes due to low volumetric density. Alternatively, storing substantial amounts of renewable energy in chemical bonds is gaining attention for on-station hydrogen delivery such as fuel cell-powered applications. For this reason, liquid organic hydrogen carriers (LOHCs) have been investigated as potential candidates based on their ability to store, transport and deliver hydrogen. Formate compounds (MHCO2, M= Na+, K+, NH4+) have been investigated as promising options among various LOHCs due to their benign nature, ease of transportation and ability to reform under mild conditions. Moreover, considering a carbon-negative approach and the potential benefit of liquid-based hydrogen storage with ΔG0r ∼0 kJmol−1, the formate-bicarbonate cycle offers a substantial advantage of storing enormous amounts of renewable energy in the chemical bonds of formate compounds for on-station hydrogen delivery. Although a continuous heterogeneous catalytic reactor is a preferred configuration for constant and stable hydrogen production, formate reforming has been hindered by catalyst deactivation in thermo-catalytic systems. This work shows the potential of using potassium formate under caustic aqueous phase electrochemical reforming (CAPER) for onboard continuous hydrogen supply. In aqueous conditions, potassium formate has been considered the ideal candidate as it yields the highest volumetric energy density (29.3 gH2/L of solution) compared to other formate compounds. Our preliminary results indicate that CAPER of potassium formate produced hydrogen with 100% faradic efficiency at a low operating cell potential of 0.189 V under ambient conditions while a decrease in kinetic overpotentials improved the hydrogen production rates with an increase in temperature up to 80 °C. Pd/C anode showed stable hydrogen production under ambient and high-pressure (60 Bar) conditions. Moreover, we also found that membrane-less electrochemical reforming of potassium formate under milder operating conditions (0.5 V and 80 °C) achieved a lower heating value efficiency () of 67% with an energy requirement of <20 kWh/kg. A comparative analysis of performance metrics showed that a membrane-less electrochemical reforming of potassium formate has an operating voltage requirement of over six times lower than a commercial electrolyzer and more than five times lower energy consumption. We expect that, under the optimized reactor conditions, our technology can meet the technical targets for liquid alkaline electrolyzers defined by the Department of Energy (DOE). Overall, the current approach offers an opportunity to integrate CAPER of potassium formate to run the bicarbonate-formate cycle for sustainable hydrogen production and grid power-balancing applications.