2025 AIChE Annual Meeting
(554a) Catalyzed Carbonization of Polyetherimide/Graphite Nanocomposites: The Role of Transition Metal Catalyst in Char Formation and Its Chemical Structure
Authors
Masoud Salavati - Presenter, University of Mississippi
Mohammed Majdoub, University of Mississippi
Oussama Oulhakem, University of Mississippi
Dineshkumar Sengottuvelu, University of Mississippi
Mine G. Ucak-Astarlioglu, ERDC
Ahmed Al-Ostaz, University of Mississippi
Sasan Nouranian, University of Mississippi
Polyetherimide (PEI) has potential to be used as precursor for ultraporous carbon molecular sieve membranes and carbon foams thanks to its favorable char formation characteristics under controlled pyrolysis conditions. Addition of a carbonaceous nanofiller, such as graphite (Gr), further improves the char yield by embedding highly ordered sp2 carbon structures in the bulk of the PEI matrix to act as a template for inducing further carbon order, i.e., sp2 (crystalline) versus sp3 (amorphous or disordered) carbon, in the char structure during carbonization. Furthermore, it can mechanically stabilize the resulting membrane structure. To further induce carbon order in the char yield, transition metal catalysts may be added to the PEI/Gr nanocomposite formulation. These catalysts are known to promote graphitization during the nanocomposite pyrolysis under an inert atmosphere; however, they may interact with graphite in either synergistic or antagonistic way. Also, the pyrolysis conditions play a critical role in the actual chemical pathway taken during catalyst-promoted graphitization reaction. All these variables make the prediction of the quantity and quality of the char yield form different applications very complicated and mostly unknown.
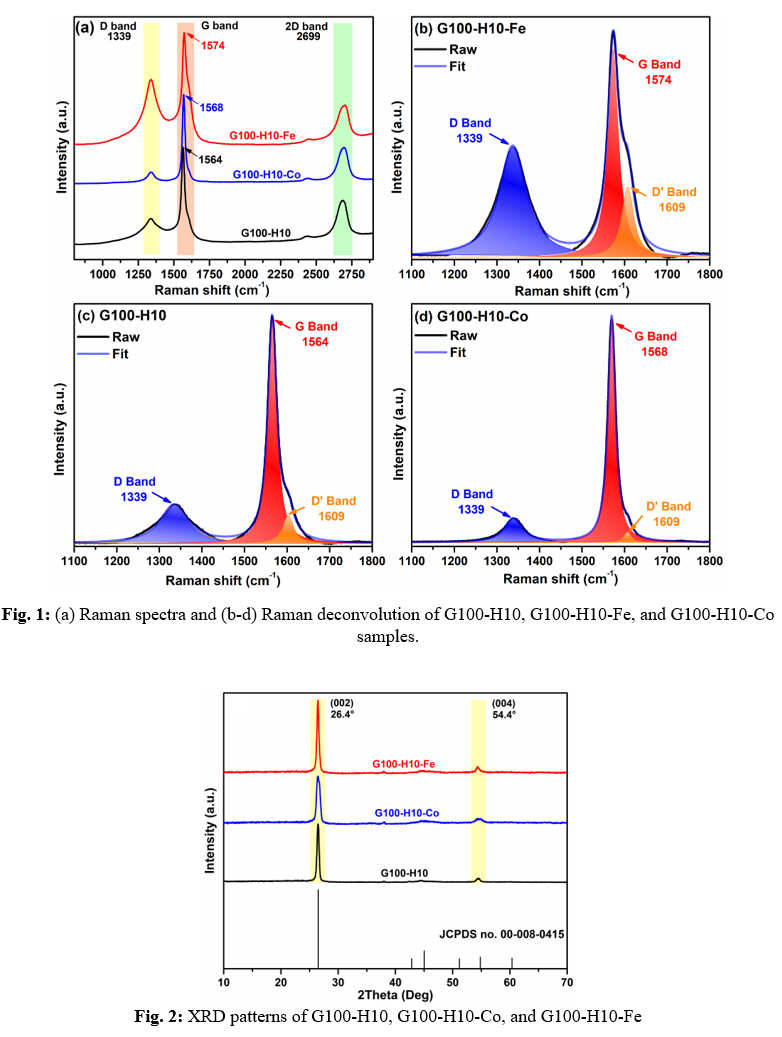
To address the above lack of knowledge and to gain a fundamental understanding of the interplay between graphite and transition metal catalysts (Fe, Co, and Ni) in the char formation of the PEI precursor and its chemical structure, an I-optimal response surface design was used to investigate the effects of Gr content (0-100 parts per hundred resin or phr), catalyst type (Fe, Co, and Ni), and heating rate during thermogravimetric analysis (10-20°C/min) on PEI-normalized char yield and PEI/Gr-normalized char yield. A comprehensive thermogravimetric analysis of 25 experimental runs yielded two statistically significant predictive models that were further validated to confirm the accuracy of the models. Based on the criterion of maximum char yield, further numerical optimization revealed the highest char yield for the combination of high Gr content (100 phr), Fe or Co (similar performance), and low heating rate (10°C/min) (designated G100-H10-Fe or G100-H10-Co) versus the neat PEI (G0-H10). However, beyond achieving the highest char yield, another objective of this study was to understand the effects of Fe and Co on the resulting carbon structure (ordered versus disordered) in the chars versus those observed for the neat PEI (G0-H10) and PEI/Gr (G100-H10). For this purpose, comprehensive structural and morphological analyses were performed on the above optimized formulations using Raman spectroscopy, X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and scanning electron microscopy (SEM). Raman spectroscopy was employed to evaluate the structural evolution and degree of graphitization in the samples (Fig. 1). The analysis mainly focused on the shift of the G band, the intensity ratio of the D to G band (ID/IG), and the full width at half maximum (FWHM) of the D band, providing insight into the extent of disorder induced by metal incorporation. The G band exhibited a progressive shift towards higher Raman shifts with metal addition. Specifically, G100-H10 displayed the G band at 1564 cm⁻¹, while G100-H10-Co and G100-H10-Fe exhibited shifts to 1568 cm⁻¹ and 1574 cm⁻¹, respectively. This systematic upshift suggests a progressive stiffening of the graphitic lattice, attributed to charge transfer effects and local strain introduced by metal coordination. Additionally, The ID/IG ratio, a metric for structural disorder, revealed distinct catalytic effects of Co and Fe. G100-H10-Co exhibited the lowest ID/IG ratio (0.14), indicative of enhanced graphitization and reduced defect density, whereas G100-H10 presented a moderate ID/IG ratio (0.27), consistent with pyrolyzed carbon without a catalytic influence. Conversely, G100-H10-Fe demonstrated the highest ID/IG ratio (0.58), signifying substantial structural disorder despite the presence of Fe, which is commonly regarded as a catalyst for graphitization. This observation suggests that Fe induces a higher concentration of defects, potentially due to Fe-N or Fe-C interactions disrupting the graphitic framework rather than promoting structural order. The XRD patterns of all samples (Fig. 2), regardless of their composition, indicated a successful graphitization indicated by the presence of a prominent and well-defined diffraction peak at an angle of 2θ = 26.4°, as well as a relatively low intensity peak at 2θ = 54.4°, which corresponds to the reflection of the (002) and (004) planes in an arranged graphitic structure. The absence of new crystalline phases suggests that the incorporation of Fe and Co did not lead to the formation of distinct metal carbide or oxide phases. The XPS analysis and SEM imaging of the samples are still ongoing.
To address the above lack of knowledge and to gain a fundamental understanding of the interplay between graphite and transition metal catalysts (Fe, Co, and Ni) in the char formation of the PEI precursor and its chemical structure, an I-optimal response surface design was used to investigate the effects of Gr content (0-100 parts per hundred resin or phr), catalyst type (Fe, Co, and Ni), and heating rate during thermogravimetric analysis (10-20°C/min) on PEI-normalized char yield and PEI/Gr-normalized char yield. A comprehensive thermogravimetric analysis of 25 experimental runs yielded two statistically significant predictive models that were further validated to confirm the accuracy of the models. Based on the criterion of maximum char yield, further numerical optimization revealed the highest char yield for the combination of high Gr content (100 phr), Fe or Co (similar performance), and low heating rate (10°C/min) (designated G100-H10-Fe or G100-H10-Co) versus the neat PEI (G0-H10). However, beyond achieving the highest char yield, another objective of this study was to understand the effects of Fe and Co on the resulting carbon structure (ordered versus disordered) in the chars versus those observed for the neat PEI (G0-H10) and PEI/Gr (G100-H10). For this purpose, comprehensive structural and morphological analyses were performed on the above optimized formulations using Raman spectroscopy, X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and scanning electron microscopy (SEM). Raman spectroscopy was employed to evaluate the structural evolution and degree of graphitization in the samples (Fig. 1). The analysis mainly focused on the shift of the G band, the intensity ratio of the D to G band (ID/IG), and the full width at half maximum (FWHM) of the D band, providing insight into the extent of disorder induced by metal incorporation. The G band exhibited a progressive shift towards higher Raman shifts with metal addition. Specifically, G100-H10 displayed the G band at 1564 cm⁻¹, while G100-H10-Co and G100-H10-Fe exhibited shifts to 1568 cm⁻¹ and 1574 cm⁻¹, respectively. This systematic upshift suggests a progressive stiffening of the graphitic lattice, attributed to charge transfer effects and local strain introduced by metal coordination. Additionally, The ID/IG ratio, a metric for structural disorder, revealed distinct catalytic effects of Co and Fe. G100-H10-Co exhibited the lowest ID/IG ratio (0.14), indicative of enhanced graphitization and reduced defect density, whereas G100-H10 presented a moderate ID/IG ratio (0.27), consistent with pyrolyzed carbon without a catalytic influence. Conversely, G100-H10-Fe demonstrated the highest ID/IG ratio (0.58), signifying substantial structural disorder despite the presence of Fe, which is commonly regarded as a catalyst for graphitization. This observation suggests that Fe induces a higher concentration of defects, potentially due to Fe-N or Fe-C interactions disrupting the graphitic framework rather than promoting structural order. The XRD patterns of all samples (Fig. 2), regardless of their composition, indicated a successful graphitization indicated by the presence of a prominent and well-defined diffraction peak at an angle of 2θ = 26.4°, as well as a relatively low intensity peak at 2θ = 54.4°, which corresponds to the reflection of the (002) and (004) planes in an arranged graphitic structure. The absence of new crystalline phases suggests that the incorporation of Fe and Co did not lead to the formation of distinct metal carbide or oxide phases. The XPS analysis and SEM imaging of the samples are still ongoing.

