2025 AIChE Annual Meeting
(391q) Catalytic Pyrolysis of Light Hydrocarbons to Light Olefin: Process Modelling Method for Predictive and Optimization
Authors
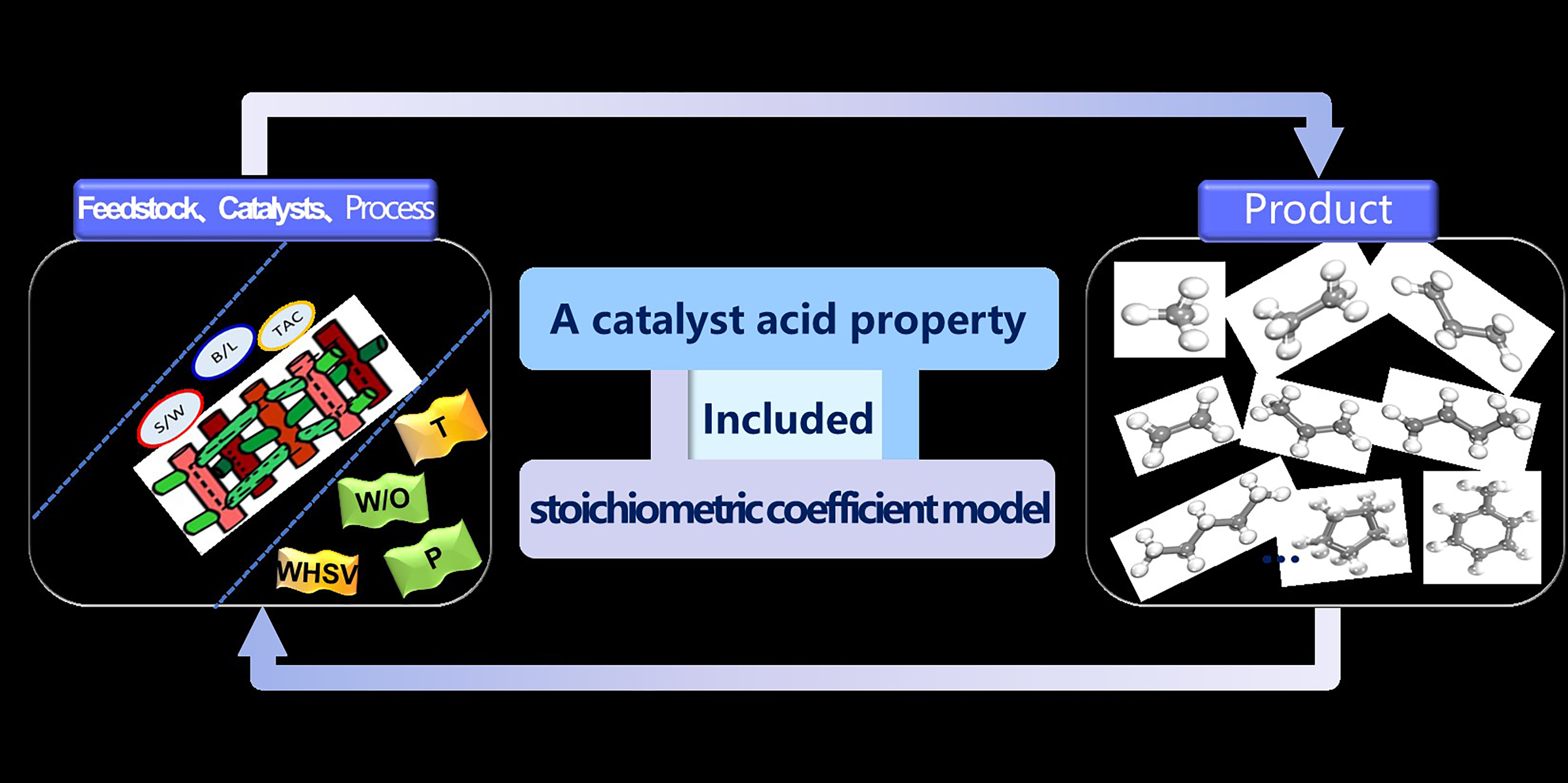
To tackle the complexity of light hydrocarbon catalytic pyrolysis systems, this research investigates the interplay between alkane and naphthene cracking mechanisms via first-order reaction kinetics, establishing a quantitative correlation between cracking activity and the average carbon number of hydrocarbon components as well as naphthene content. A stoichiometric coefficient database for cracking products under various catalytic systems was constructed by incorporating stoichiometric coefficients to describe evolving product distributions. Furthermore, a quantitative relationship between cracking product stoichiometric coefficients and catalyst acid properties was established based on their empirical correlations. Finally, the stoichiometric reaction was used to describe the reaction process, and the stoichiometric coefficient was used to describe the distribution of pyrolysis products, coupled with the catalyst property parameters.
This model enables accurate predictions of molecular-level cracking product distributions (<2 wt% absolute error) across diverse catalytic systems and identifies optimal catalyst acid property ranges for maximizing light olefin yields. By achieving bidirectional control from feedstock to products and from products to catalysts, this methodology broadens the application of catalytic pyrolysis models in catalyst design and selection, offering a model-based approach for intelligent process control.