2025 AIChE Annual Meeting
(319d) Catalytic Insights into Ruthenium-Boron Nitride Nanotubes (Ru/BNNTs) for Low-Temperature Ammonia-to-Hydrogen Conversion
Authors
Tae Jin Kim - Presenter, Stony Brook University
Dolly Yadav, NAiEEL Technology
Thomas You-Seok Kim, University of Iowa
Chi Ho Lee, Texas A&M University
Insoo Ro, University of California, Santa Barbara
Eunkwang Park, NAiEEL Technology
Gihan Kwon, Brookhaven National Laboratory
Joseph Kwon, Texas A&M University
Jaewoo Kim, NAiEEL Technology
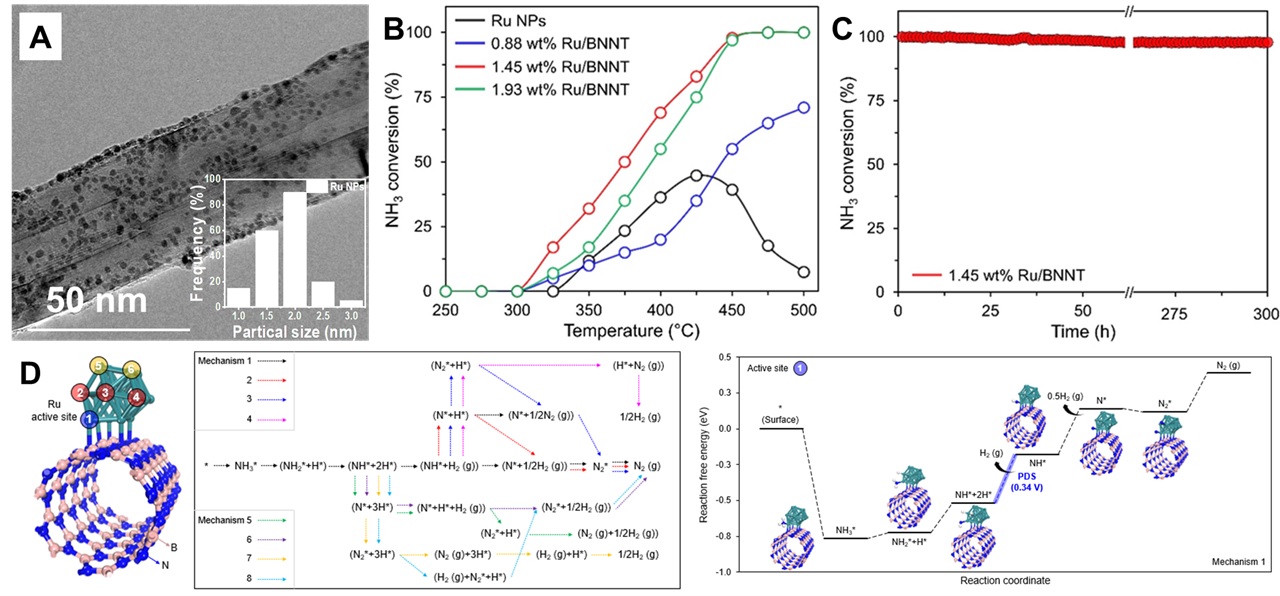
In context to the energy transition from fossil fuels to renewables and low-carbon energy resources, hydrogen (H2) production from ammonia (NH3) has emerged as a promising route for long-term and sustainable energy storage [1-3]. Tailoring metal nanoparticle-surface support interactions offers a promising approach to enhancing the activity and durability of high-performance catalysts for energy production. Herein, we present boron nitride nanotubes decorated with ruthenium nanoparticles (Ru/BNNT) as a catalyst for the low-temperature conversion of NH₃ to H₂. Among all tested samples, Ru/BNNT with 1.45 wt% Ru exhibited the highest catalytic performance, achieving ~99.99% NH₃ conversion at 475 ℃ (Fig. 1(A, B)). Notably, even at the lowest Ru content (0.88 wt%), Ru/BNNT achieved >50% NH₃ conversion at 450 ℃, underscoring its economic potential. In-situ HR-TEM studies provided insights into the thermochemical stability of Ru/BNNT, demonstrating minimal sintering at 1150 ℃ and stable catalysis for over 300 hours (Fig. 1(C)), with ~95.25% NH₃ conversion. Furthermore, density functional theory (DFT) calculations identified the most favorable mechanism among eight possible pathways, revealing the critical role of dual Ru sites in separately facilitating NH₃ decomposition and H₂ desorption (Fig. 1(D)). The current work offers fundamental insights into metal nanoparticle-surface support interactions, driving scientific interest in the synthetic utilization of BNNT for advancing fuel cell and energy technology.
Figure 1. (A) TEM image of 1.45 wt% Ru/BNNT, (B) Catalytic activity tests for NH3-to-H2 production, (C) Long term stability experiment for >300 h at 500 °C, (D) DFT based mechanistic insights into Ru/BNNT for NH3-to-H2 catalysis.
References
[1] R. Schüth et al., Energy Environ. Sci. 5 (2012) 6278. [2] K. Chen et al., Energy Fuels 35 (2021) 11693. [3] L.A. Spatolisano et al., Ind. Eng. Chem. Res. 62 (2023) 10813.