2025 AIChE Annual Meeting
(657g) Catalytic Degradation of Hdpe and Ldpe over Zeolite Beta in Cetane
Authors
5.0 g of HDPE (Mw~174.00) or LDPE (Mw~216,000), 20 g of solvent, and 0.25 g or 1.0 g of catalyst were added to a 100 cc batch reactor and the reaction was carried out. The catalysts used were beta zeolite and amorphous silica-alumina (ASA). The reaction temperature was 360, 380 or 400 oC and the reaction periods were 30, 60 or 90 min. n-C16, cetane, was used as solvent. The products obtained were analyzed by GC-FID, GC×GC-MS, and distillation GC. After the degradation test, the catalyst was rinced to remove residual undegraded PE with tetrachlorobenzene and the amount of carbon deposition was determined by TG-DTA. In addition, the molecular weight of the undegraded PE was measured by high temperature GPC.
To study the differences in the decomposition activity of ASA and zeolite beta for HDPE and LDPE, an activity test was performed at 400 oC for 60 min. As a result, when Beta was used, the order of PE conversion was HDPE>LDPE, while when ASA was used, they were similar. With beta zeolite, a high yield of C3~C9 aliphatic hydrocarbons was observed, while when ASA was used, relatively higher yields of heavy hydrocarbons were obtained. In addition, when the samples were pyrolyzed for comparison, it was found that LDPE had a higher level of conversion than HDPE. These results suggest that HDPE, which has fewer branches, has better access to the micropores of beta zeolite, and decomposed there.
We conducted an activity test with beta and ASA at temperatures below 380 oC, where thermal decomposition is unlikely to occur. For beta, the conditions were 360 oC for 30 min and for ASA, 380 oC for 60 min. The amount of Beta used was 0.25 g, and the amount of ASA used was 1.0 g. This was done to study the molecular weight of the products and the undecomposed PE at similar levels of PE conversion. The molecular weight of undecomposed PE was also compared with the results of thermal decomposition. Regarding the PE conversion, when beta was used, the PE conversion was HDPE>LDPE, whereas when ASA was used, HDPE=LDPE.
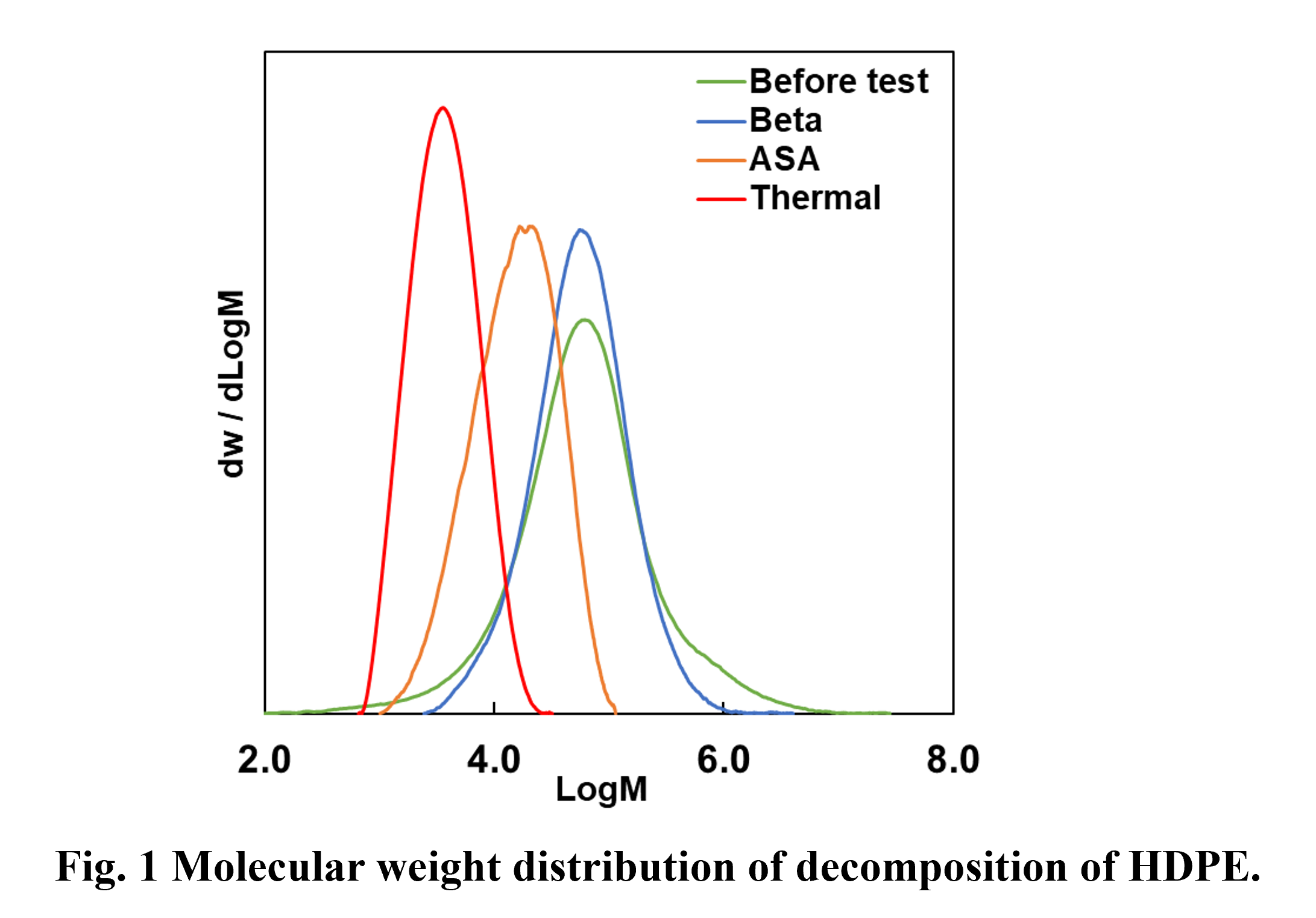
In addition, as shown in Fig. 1, even at low conversion rates, the molecular weight of the PE decreases significantly during thermal cracking. On the other hand, when Beta zeolite was used, it was found that even at high conversion rates, the molecular weight of the remaining polymer decreased only slightly, especially in the case of HDPE. From these results, it was inferred that when HDPE decomposition begins with Beta zeolite, it progresses rapidly to low-molecular-weight liquid products.
From these results, it was suggested that PE is effectively degraded by the acid sites of zeolite. In addition, the results of the reactions using ASA and beta indicate that the decomposition of HDPE, which has few branches, occurred in the micropore of zeolite beta.