2025 AIChE Annual Meeting
(382bm) Catalyst Engineering for Electrochemical Transformations
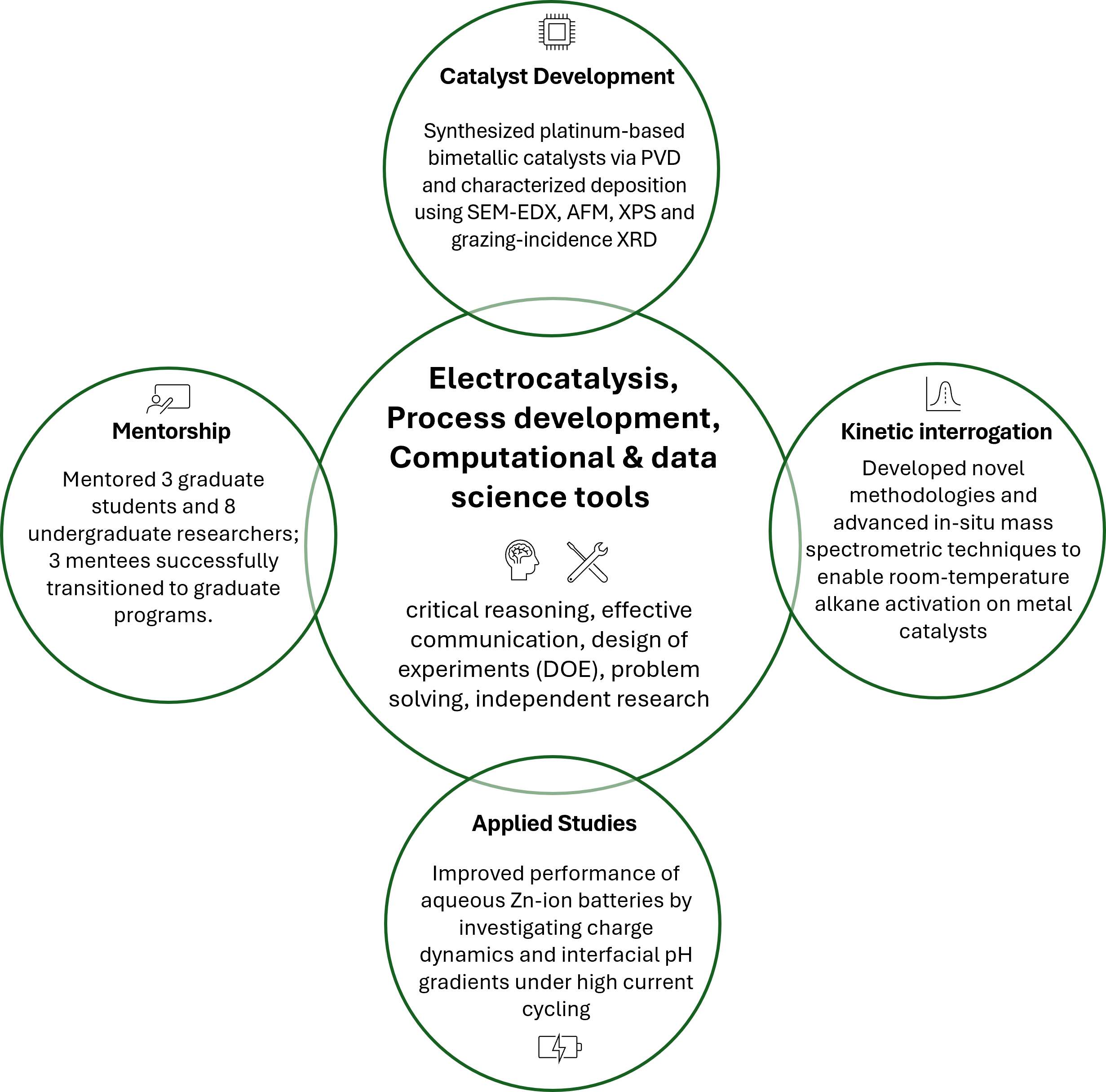
Fundamental advances at the intersection of catalysis, material science, and engineering will be pivotal in developing transformative solutions for decarbonizing the chemical and energy industries. For instance, various industrial processes – such as alkane dehydrogenation and alkene epoxidation – rely on commercial thermal routes that are both energy- and carbon-intensive, highlighting the need for breakthroughs in engineered catalysts to make these transformations more sustainable. [1] As part of my PhD at Purdue University, I have developed expertise in electrocatalysis, with a focus on elucidating mechanistic pathways and performing kinetic investigations to determine rate parameters in electrocatalytic systems. In the next stage of my career, I aim to utilize my expertise and transferable skills, such as problem-solving and critical reasoning developed during my graduate education, to drive impactful solutions in the chemical and energy industry.
Research Summary
Developing sustainable pathways to enable chemical transformations for decarbonized chemical manufacturing. For instance, Pt electrocatalysts can activate alkanes, but selective transformation beyond CO2 has been a significant challenge. My thesis focuses on probing Pt-based catalysts and tailoring reaction microenvironments to enable room-temperature activation to higher-value products such as propylene.
Abstract
My PhD research focuses on room temperature electrocatalytic activation of propane on Pt-based electrocatalysts. Conventional high-temperature thermal processes used for such endothermic activation of paraffinic C—H bonds are highly energy-intensive and contribute significantly to greenhouse gas emissions. This underscores the need for bond activations at lower temperatures, where an electrochemical pathway can be utilized to modulate the Gibbs free energy of reaction by using electrochemical potential. Our work addresses the mechanistic challenges of propane activation at room temperature, where Pt exhibits the unique ability to enable this process but tends to drive complete oxidation rather than partial oxidation to value-added products. We demonstrated that, under room-temperature aqueous acidic conditions, propane adsorption is favored at electrode potentials free from water-derived adsorbate species. Furthermore, using on-line electrochemical mass spectrometry (ECMS), we identified propane- derived surface adsorbates as deeply dehydrogenated hydrocarbons retaining an intact C₃ backbone (C₃H₍₈₋ᵧ₎). [2]
Despite these mechanistic insights, significant challenges remain in fully understanding the kinetics of propane activation and the factors that govern selectivity and conversion to desired products. To address this, we extended our analysis to investigate propane adsorption and desorption kinetics on Pt using a coulometric approach. We developed a methodology to benchmark activation kinetics, leveraging the current transient signature (observed as propane is introduced in the electrochemical cell) as a means to extract rate parameters in the absence of continuous activation. This is the work that I will be presenting at the 2025 AIChE annual meeting in Boston. Lastly, our current ongoing work focuses on modifying Pt-based catalysts with tailored properties to enable the activation of previously inaccessible chemical transformations.
Selected Publications
- Bhadouria, A., Biswas A., Tackett, B.M., “Electrocatalytic Transformations of C2 and C3
Hydrocarbons: Bridging from the Past to the Future.” ACS Catalysis, 15, 8, 2025, 6296–6314.Ashutosh Bhadouria - Bhadouria, A., Heil J.N., Parab D.E., Greeley J., Tackett, B.M., “Propane Activation on Pt
Electrodes at Room Temperature: Quantification of Adsorbate Identity and Coverage.” Angewandte
Chemie International Edition, 2025, 64 (11), e202421613.