2025 AIChE Annual Meeting
(254e) Brønsted Acid Site Formation in Hydrodeoxygenation on Metal Oxide Surfaces: The Enigmatic Case of MoO3
Authors
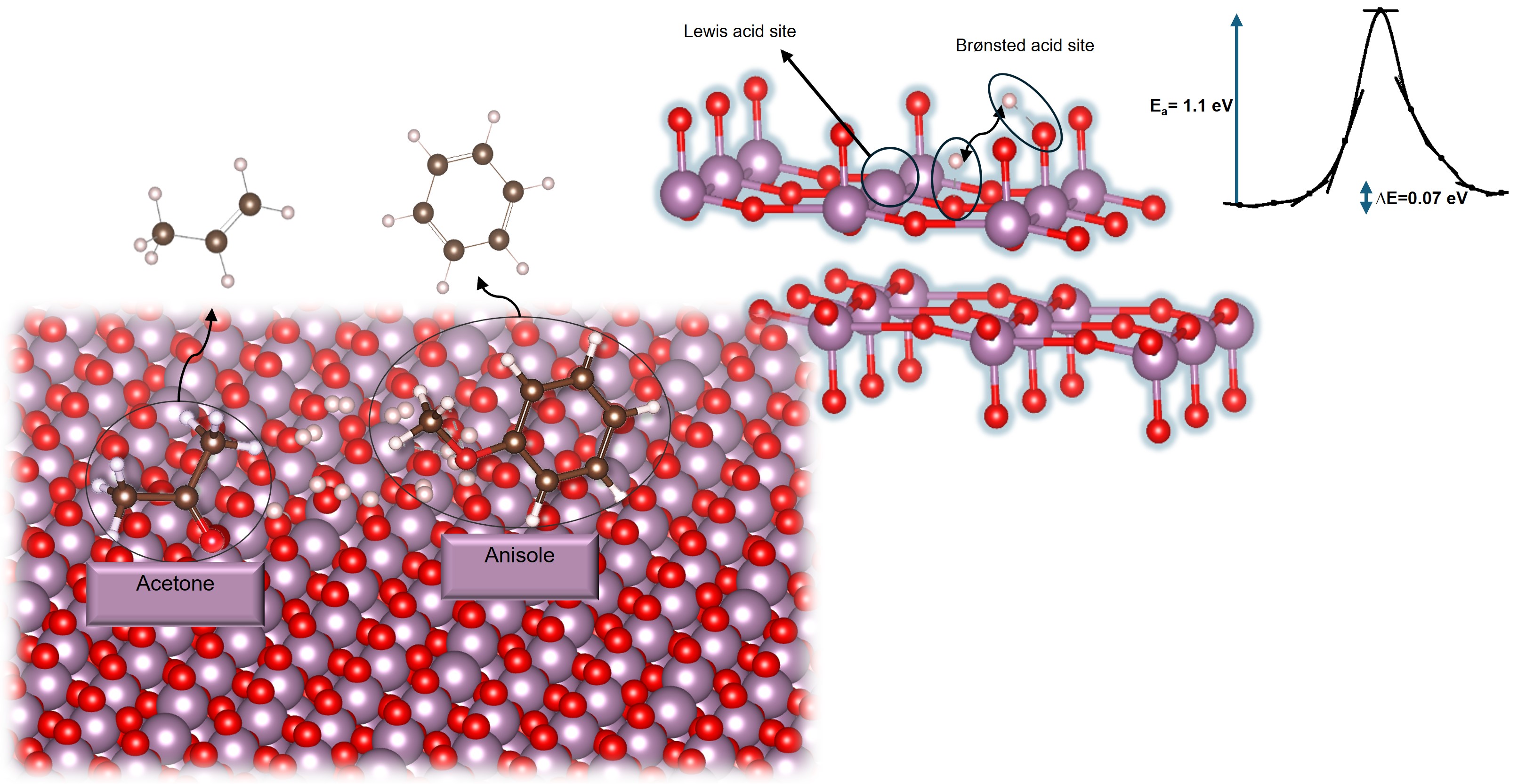
In this presentation, we share our recent computational results comparing acetone and anisole HDO mechanisms on α-MoO₃(010). Our DFT calculations highlight the role of oxygen vacancies in a reverse Mars-van Krevelen mechanism but initially overlooked the importance of surface hydroxyls on the mechanism. We therefore expanded our calculations with an in-depth study of H adsorption to better understand the true active site, showing that asymmetric oxygen favors hydroxyl formation both thermodynamically and kinetically. It was observed that hydrogen spillover to neighboring sites is facile due to low activation barriers (between 0.6 and 1.1 eV for hydrogen travel between terminal and bridge oxygens). These findings aid in better understanding the surface post-reduction and refining adsorption models for carbonyl-containing compounds, showing the importance of using the correct surface and improving the fidelity of our computational models with our experimental HDO kinetics experiments, enhancing the explanatory and predictive power of computations.
References:
- Kohler, A.J. et al. ACS Catal. 2023, 13, 22, 14813–14827.
- Ikeda, Y. et al. Phys. Chem. C 2022, 126, 17, 7728–7738.
- Prasomsri, T. et Energy Environ. Sci. 2013, 6 (6), 1732–1738.