2025 AIChE Annual Meeting
(410g) Bringing Rationality into the Development of Low Cost and Active Photocatalyst for CO2 Conversion to Methanol - Bridging Experiments and First-Principles Calculations
Authors
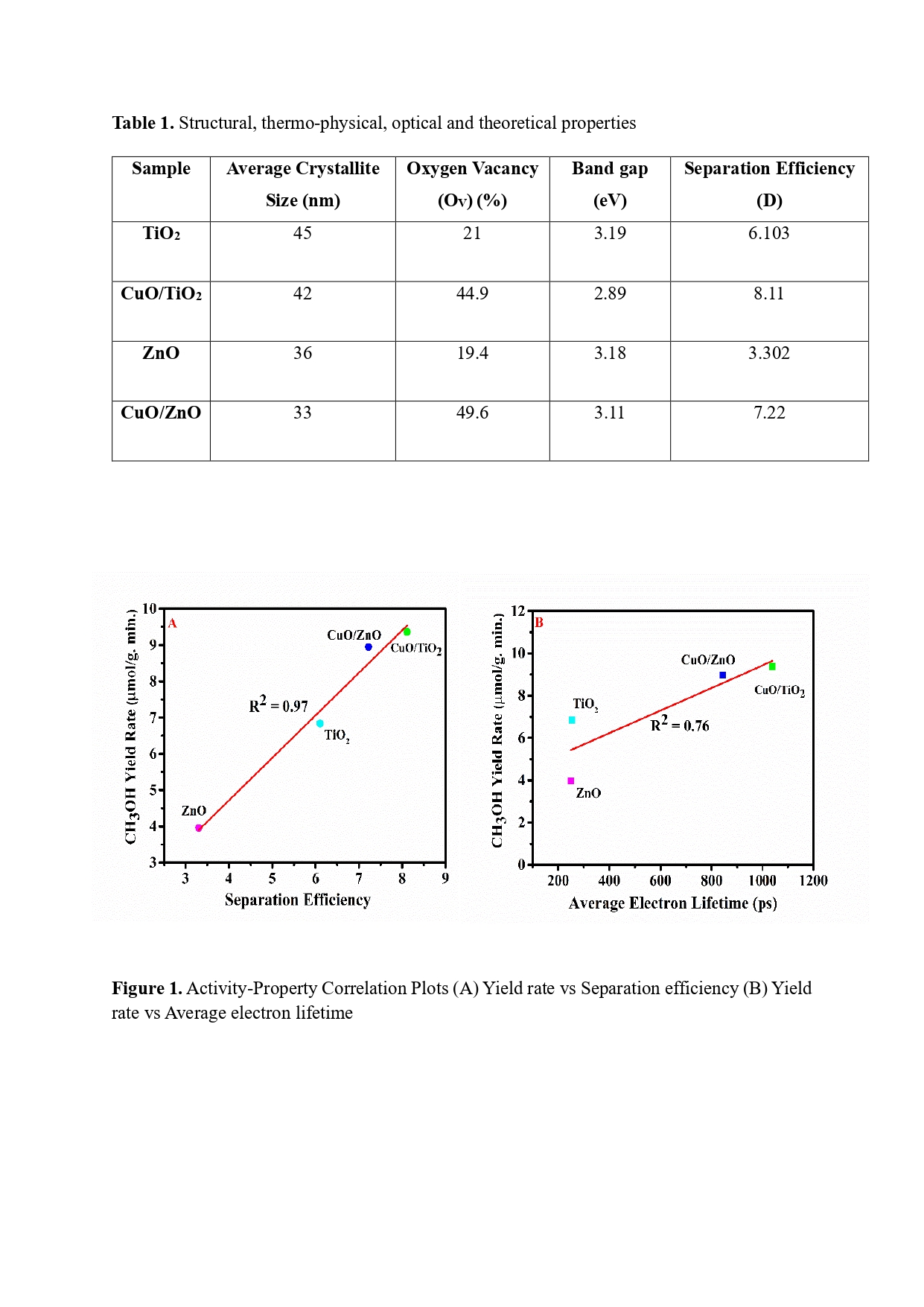
Both CuO/TiO2 and CuO/ ZnO heterostructure catalysts were fabricated impeccably, and during photoinduced reactions, both yielded additional methanol compared to TiO2 and ZnO, respectively, implying successful creation of heterojunction photocatalysts. The higher yield of CuO/TiO2 than CuO/ZnO demonstrated that TiO2 is a prominent choice than ZnO due to more narrowing of band gap, better charge separation and lower recombination rate. Finally, methanol yield rate when correlated with separation efficiency and average electron lifetime, it produced almost linear dependency, which are representative of structure-activity relationships. Moreover, the increased oxygen vacancy by adding CuO enabled more CO2 activation, leading to higher photo-reactivity. Therefore, this study delved into improved understanding on the intrinsic properties of the photocatalysts from experimental characterization and theoretical calculations and finally used them to explain the activity trend. Overall, this approach marks as a rationalised way forward for exploring efficient methanol forming catalysts by photocatalytic CO2 reduction.