2025 AIChE Annual Meeting
(185q) Biostable PbS/CdS/ZnS Quantum Dots for High Dynamic Range Swir Imaging
Fluorescence imaging with contrast agents is a standard tool used to label specific tissues in vivo for various biomedical applications because of its low cost of non-ionizing radiation. This imaging modality, however, often uses visible and near-infrared (NIR-I) fluorophores whose emitted light has limited penetration due to light scattering through tissue and poor signal-to-background ratio due to autofluorescence, and poor photostability from rapid photobleaching.[1, 2] To address these difficulties, we previously utilized short-wave infrared (SWIR, or NIR-II; 1000-1700 nm) emitting lead-sulfide quantum dots with a cadmium-sulfide shell (PbS/CdS core-shell QDs) to label vasculature and lymphatic drainage pathways.[3] However, quantitative imaging was limited by nanoparticle photostability and low dynamic range of our InGaAs SWIR camera. Poor photostability limits correlation of measured image intensity to true fluorophore concentration, and low camera dynamic range hinders simultaneous quantitation of QDs in organs and tissues with wide ranges of uptaken QD concentrations.
Methods
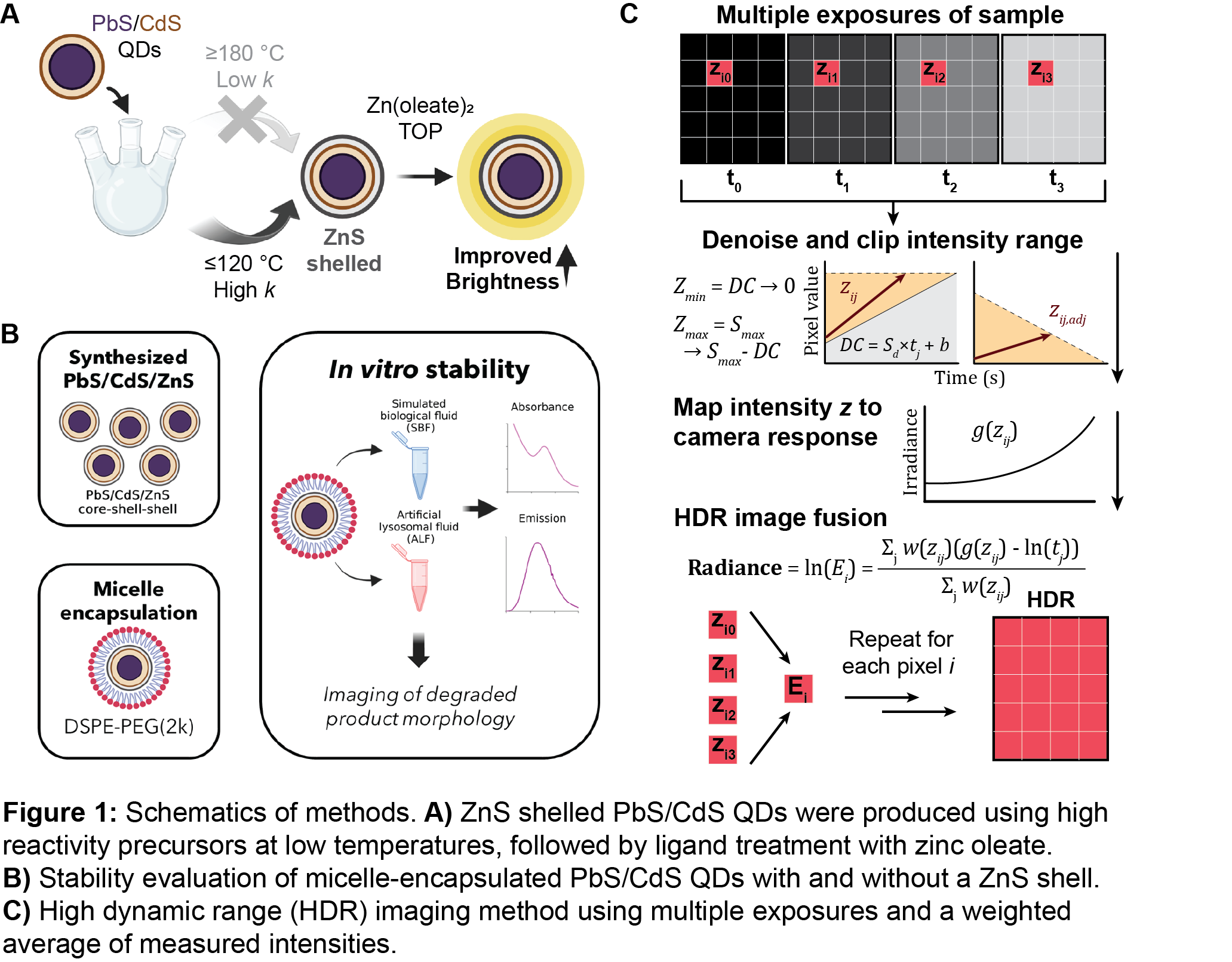
To enhance photostability of PbS/CdS QDs we added an additional outer protective zinc-sulfide (ZnS) shell layer. PbS/CdS QDs were unstable at high temperatures, which required us to explore and adapt several zinc and sulfur precursor candidates for lower-temperature reactions (Figure 1A). These precursors included diethyl zinc with bis(trimethylsilyl)sulfide, zinc oleate with various disubstituted thioureas, and a single-source precursor, zinc diethyldithiocarbamate.[4, 5] We evaluated QD stability by first encapsulating plain PbS/CdS and ZnS-shelled QDs in 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-(polyethylene glycol)-2000 (DSPE-PEG(2k)) lipid micelles to make the QDs water-soluble. These DSPE-PEG(2k)-encapsulated QDs were then treated with simulated biological fluid (SBF, extracellular space-like, neutral pH) and harsher artificial lysosomal fluid (ALF, lysosomal-like, acidic pH), and we observed structural and optical changes (Figure 1B).
Then, using high-dynamic range (HDR) imaging techniques with multiple exposures,[6, 7] we imaged SWIR-emitting QDs in phantoms and mice at a high range of concentrations. We dynamically denoised high-noise SWIR images and clip irregular intensities to accurately represent linear increases intensity with exposure time. We then performed image fusion, which, for each pixel, determines the true irradiance at the camera sensor by mapping exposure-adjusted intensities to a camera response curve and taking a weighted average that prioritizes intensities with a high signal-to-noise ratio (Figure 1C).
Results and Discussion
For each explored zinc and sulfur precursor combination, we optimized precursor addition cadence and stoichiometry to produce sufficiently thick ZnS shells (at least 1 molecular layer) on PbS/CdS QD cores. QD brightness decreased considerably with ZnS shell addition, which we recovered to viable levels for imaging by performing a post-synthetic ligand treatment with zinc oleate and trioctylphosphine. We selected the optimal synthetic method for future work by considering final QD optical properties and ZnS shell thickness, which led us to using zinc diethyldithiocarbamate in a heat-up reaction scheme.
The resulting PbS/CdS/ZnS core-shell-shell particles maintained compositional and photoluminescence stability in physiological media. Both particles were sufficiently stable in SBF. Sizing degraded particles with transmission electron microscopy (TEM) in tandem with elemental analysis showed that the ZnS shell significantly limited degradation in ALF and other acidic environments. Moreover, ZnS shelled QDs maintained a consistent fluorescent signal overtime and were much brighter in aqueous media because the ZnS shell passivates the QD surface. These compositional and photostability improvements were reflected in mouse studies where particles with a ZnS shell retained their fluorescent signal for much longer (up to 7 days) after intravenous administration, whereas particles without a ZnS shell lost most of their signal within 24 hours.
With our improved fluorophores in hand, we moved towards improving SWIR image dynamic range. With our HDR method, we saw up to a ~15 dB improvement in dynamic range and ~5 dB improvement in image contrast-to-noise ratio throughout both phantom and mouse imaging. Given that we record an intensity for each pixel that is not saturated or noisy, we are able to produce HDR images with good quantitative accuracy that is not limited by our camera’s capabilities.
This work uses improvements in both fluorophore quality and image processing techniques to advance toward true quantitation of fluorophores for chemical imaging of biological systems.
References
- 1. Sun, Y., X. Zhong, and A.M. Dennis, “Minimizing near-infrared autofluorescence in preclinical imaging with diet and wavelength selection,” Journal of Biomedical Optics, 28 (9), p. 094805 (2023).
- 2. Zhao, J., D. Zhong, and S. Zhou, “NIR-I-to-NIR-II fluorescent nanomaterials for biomedical imaging and cancer therapy,” Journal of Materials Chemistry B, 6 (3), pp. 349–365 (2018).
- 3. Zhong, X., A. Patel, Y. Sun, A.M. Saeboe, and A. Dennis, “Multiplexed Shortwave Infrared Imaging Highlights Anatomical Structures in Mice,” Angewandte Chemie International Edition, 63, p. e202410936 (2024).
- 4. Hendricks, M.P., M.P. Campos, G.T. Cleveland, I. Jen-La Plante, and J.S. Owen, “A tunable library of substituted thiourea precursors to metal sulfide nanocrystals,” Science, 348 (6240), pp. 1226–1230 (2015).
- 5. Wang, H., H. Nakamura, M. Uehara, Y. Yamaguchi, M. Miyazaki, and H. Maeda, “Highly Luminescent CdSe/ZnS Nanocrystals Synthesized Using a Single-Molecular ZnS Source in a Microfluidic Reactor,” Advanced Functional Materials, 15 (4), pp. 603–608 (2005).
- 6. Debevec, P.E., and J. Malik, “Recovering high dynamic range radiance maps from photographs,” Proceedings of the 24th annual conference on Computer graphics and interactive techniques, ACM Press/Addison-Wesley Publishing Co. (1997), 369–378.
- 7. Vinegoni, C., P. Fumene Feruglio, and R. Weissleder, “High Dynamic Range Fluorescence Imaging,” IEEE Journal of Selected Topics in Quantum Electronics, 25 (1), pp. 1–7 (2019).