2025 AIChE Annual Meeting
(276a) Biomaterial Facilitated on-Demand Delivery of Mitochondria for Modulating the Bioenergetic State of Cells
Authors
With the breakthrough observation that mitochondria are donated and received by cells to modulate cell behavior [3], mitochondria transplantation has been researched as a strategy to manufacture more potent cell-based therapeutics or promote tissue repair [2]. In musculoskeletal models, exogenous mitochondrial transfer has supported skeletal muscle regeneration in BaCl₂-induced injury [4] and facilitated bone regeneration in mouse calvarial defects [5]. However, these applications are constrained by poor uptake efficiencies and immune clearance, which limit mitochondrial bioavailability and therapeutic potency [2].
To address these challenges, we propose a two-pronged strategy: (1) enhance mitochondrial uptake via polymer-based surface functionalization, and (2) achieve controlled, localized release using matrix metalloproteinase (MMP)-cleavable hydrogel microparticles (Figure 1A). We hypothesize that mesenchymal stem cell (MSC)-derived mitochondria, delivered using this system, will augment mitochondrial transfer to myogenic and osteogenic progenitors, thereby enhancing myogenesis and osteogenesis through improved bioenergetic support.
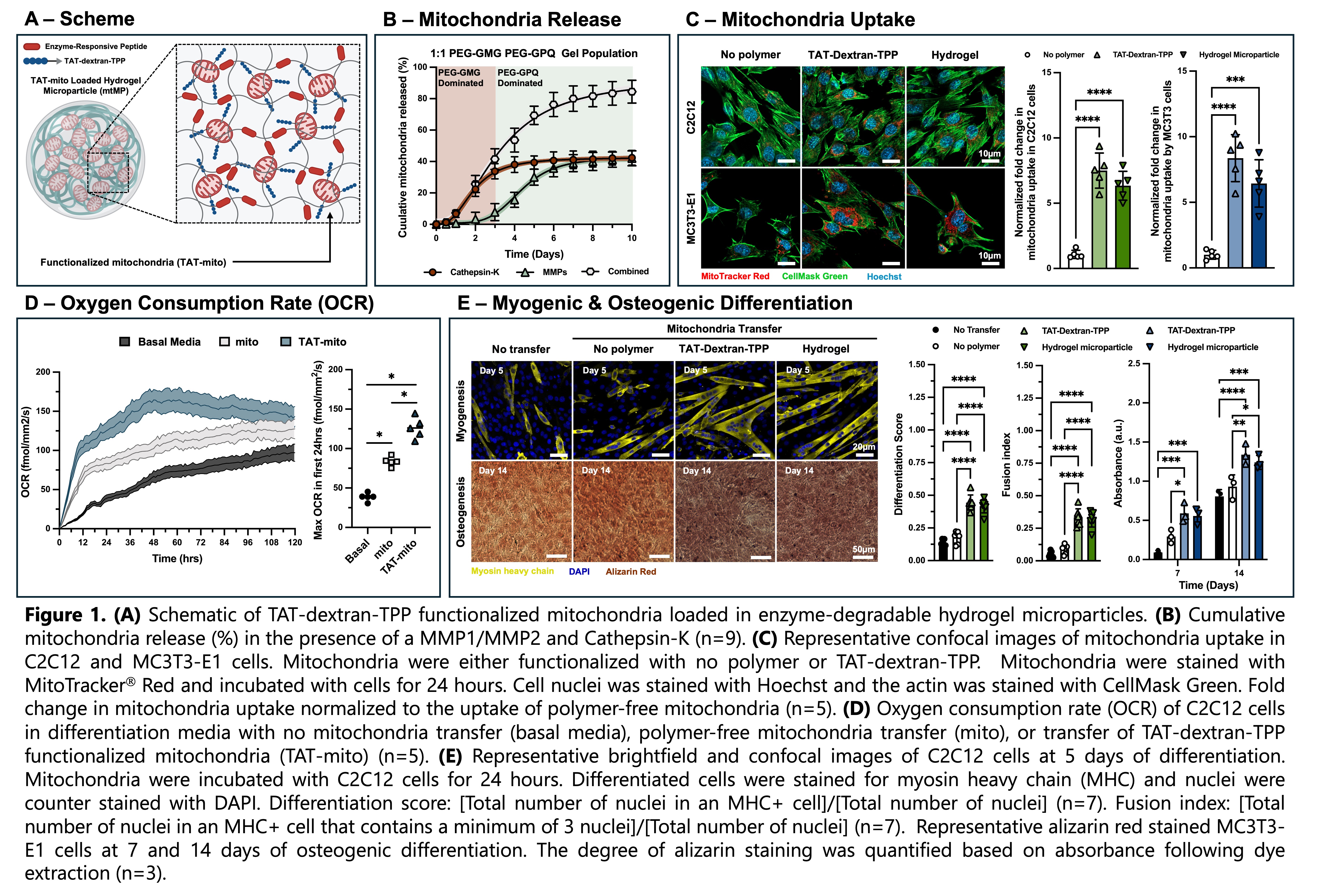
Results and Discussion. To investigate the potential for the therapeutic delivery of mitochondria, mitochondria derived from mesenchymal stem cells were encapsulated within ~90 µm enzyme-degradable hydrogel microparticles via droplet microfluidics. Controlled enzymatic degradation via MMPs or cathepsin-K facilitated gradual mitochondrial release over 5 days (Figure 1B). Post-release, the mitochondrial membrane potential was preserved, confirming organelle viability.
As previously noted, the uptake efficiency of free mitochondria is limited and highly variable. To enhance cellular internalization, we sought to functionalize MSC-derived mitochondria with a dual-functional polymer designed to (1) insert into the mitochondrial membrane, thereby affixing the polymer to the organelle surface, and (2) facilitate peptide-mediated endocytosis by recipient cells. To this end, dextran-amine was conjugated with triphenol phosphine (TPP), a mitochondria-targeting moiety commonly used in drug delivery systems, and the transactivator of transcription (TAT) peptide (QPRRRQRRKKKRG), a well-characterized cell-penetrating sequence known to enhance endocytosis. We found a 7.5-fold and 6.3-fold increase in myogenic progenitor cell (C2C12) uptake of TAT-dextran-TPP functionalized mitochondria (TAT-mito) and TAT-mito released from hydrogel microparticles respectively following 24-hour incubation compared to non-functionalized mitochondria (Figure 1C). Similarly, we found an 8.4-fold and 6.4-fold increase in osteoprogenitor cell (MC3T3-E1) uptake of TAT-mito and TAT-mito released from hydrogel microparticles respectively. Enhanced mitochondrial uptake significantly elevated oxygen consumption rate (OCR) in C2C12 cells over 48 hours, indicating upregulated OxPhos activity (Figure 1D).
We next assessed whether this bioenergetic shift translated to improved lineage-specific differentiation. Following mitochondria transfer, myogenic differentiation of C2C12 cells was performed for 5 days. We observed greater differentiation and greater fusion of myoblasts into myotubes for conditions with increased mitochondria uptake (Figure 1E). We also characterized the osteogenic differentiation of MC3T3-E1 cells post mitochondria transfer. Osteogenic differentiation of osteoprogenitor cells was performed over 14 days. At days 7 and 14, an alizarin red assay was used to stain for calcium deposition and quantified. At days 7 and 14, we observed greater calcium deposition for conditions with increased mitochondria uptake (Figure 1E).
Conclusions. From this proof-of-concept study, we were able to illustrate that mitochondria can be isolated from MSC culture, loaded into enzyme-cleavable hydrogel microparticles and released, all while preserving their bioenergetic function in vitro. Moreover, mitochondria surface modification with TAT-dextran-TPP increased internalization by C2C12 and MC3T3-E1 cells, promoting a larger degree of myogenic and osteogenic differentiation respectively. We note that while these results are most directly applicable for muscle and bone regeneration, on-demand mitochondrial release for localized cellular uptake has applications in a wide range of regenerative medicine applications.
Looking ahead, my research lab will focus on engineering novel biomaterial systems to precisely regulate mitochondrial fate post-transfer. As previously highlighted, mitochondria are highly dynamic and partake in signaling cascades beyond metabolism. As such, proper control of mitochondria fusion dynamics with the endogenous mitochondrial network and cristae restructuring has the potential to increase the potency of mitochondria transplantation and drive highly specific phenotypic changes in recipient cells. This work lays the foundation for a new class of mitochondria-based therapies with broad implications across regenerative medicine, immunomodulation, and the treatment of metabolic and degenerative diseases.
Acknowledgement. This work was supported by the Carol Ann and David D. Flanagan Professorship in BME at GT/Emory.
References.
[1] Monzel, A. S.; Enriquez, J. A.; Picard, M. Multifaceted mitochondria: moving mitochondrial science beyond function and dysfunction. Nature Metabolism 2023, 5 (4), 546-562.
[2] N. Borcherding and J.R. Brestoff. The power and potential of mitochondria transfer. Nature 2023, 623, 283-291
[3] J.L. Spees, S.D. Olson, M.J. Whitney, and D.J. Prockop. Mitochondrial transfer between cells can rescue aerobic respiration. Proceedings of the National Academy of Sciences 2006, 103 (5), 1283-1288
[4] J. Sun, H.T.J. Lo, L. Fan, T.L. Yiu, A. Shakoor, G. Li, W.Y.W. Lee and D. Sun. High-efficiency quantitative control of mitochondrial transfer based on droplet microfluidics and its application on muscle regeneration. Science Advances 2022, 8, 9245
[5] J. Suh, N.K. Kim, W. Shim, S.H. Lee, H.J. Kim, E. Moon, H. Sesaki, J.H. Jang, J.E. Kim and Y.S. Lee. Cell Metabolism 2023, 35 345-360